Breakthrough Antibody Drugs Approved in January 2025
In January 2025, multiple antibody-based therapies received global regulatory approvals, offering new hope for the treatment of various diseases. These innovative drugs span multiple therapeutic areas, including metabolic disorders, cancer, and rare diseases, showcasing the immense potential of modern biotechnology in addressing major health challenges.
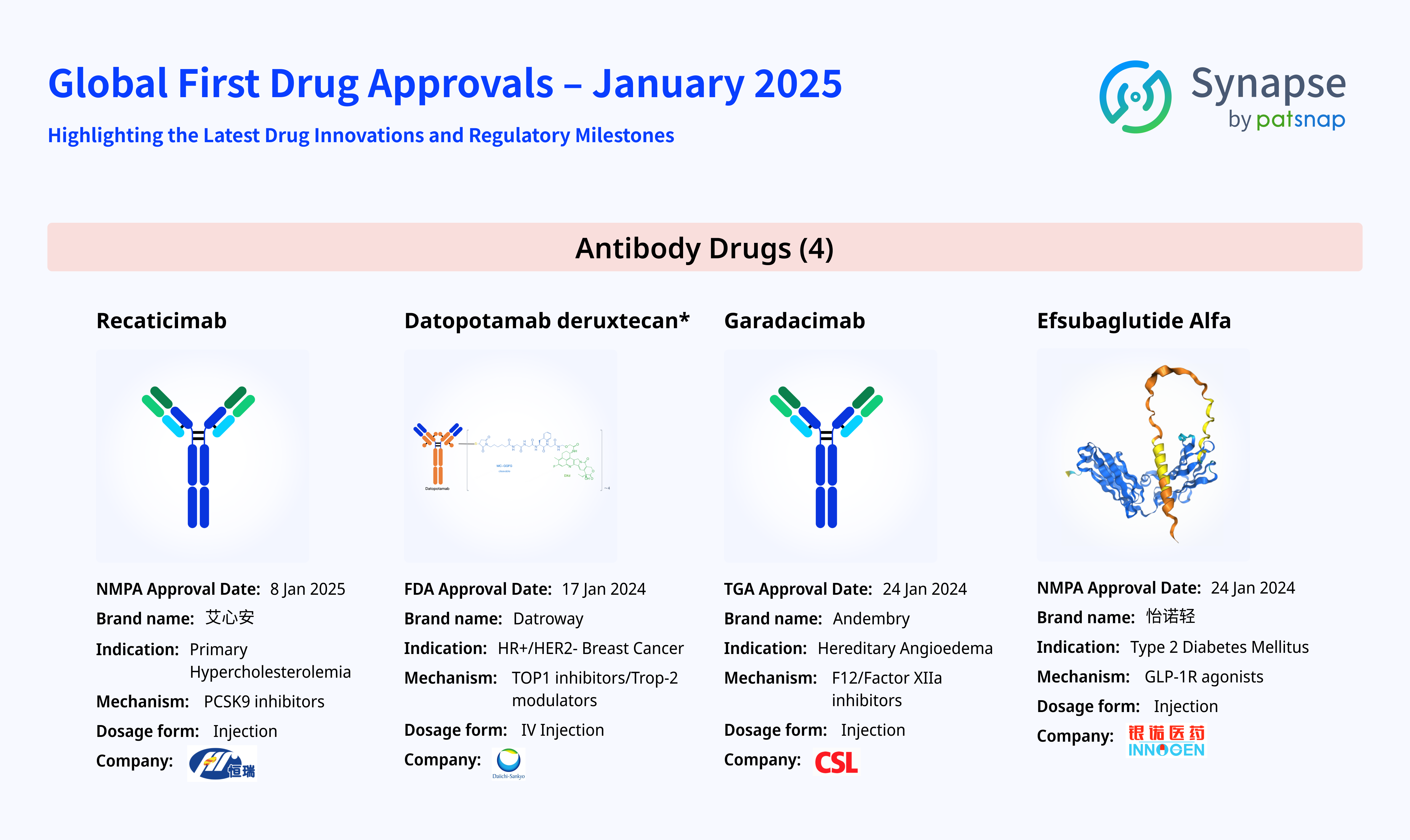
From Recaticimab, a novel treatment for cardiovascular diseases, to Datopotamab Deruxtecan, an advanced therapy for breast cancer, and Garadacimab, designed for hereditary angioedema (HAE), these breakthroughs are transforming the landscape of disease management. Additionally, Efsubaglutide Alfa, a long-acting GLP-1 receptor agonist, introduces a new treatment option for type 2 diabetes. These cutting-edge therapies are reshaping our understanding of diseases and revolutionizing treatment strategies.
Click the link below for in-depth analyses of each drug:
Recaticimab: A Novel PCSK9-Targeting Monoclonal Antibody for Hypercholesterolemia
Datopotamab Deruxtecan: A Novel ADC Targeting Trop-2 for Advanced Breast Cancer
Garadacimab: A Novel FXIIa-Targeting Monoclonal Antibody for Hereditary Angioedema
Efsubaglutide Alfa: A Novel GLP-1 Fusion Protein Revolutionizing Type 2 Diabetes
The early months of 2025 marked the launch of several milestone antibody therapies. Among them: Recaticimab (Hengrui Pharmaceuticals): A PCSK9-targeting monoclonal antibody that increases hepatic LDL receptor (LDLR) expression, enhancing low-density lipoprotein cholesterol (LDL-C) clearance. This ultra-long-acting therapy offers a groundbreaking solution for hypercholesterolemia patients. Datopotamab Deruxtecan: A Trop-2-directed antibody-drug conjugate (ADC) that has demonstrated remarkable efficacy in breast cancer and other solid tumors. Garadacimab: A novel therapy bringing new hope to hereditary angioedema (HAE) patients. By inhibiting activated coagulation factor XIIa, Garadacimab reduces the frequency of angioedema attacks, significantly improving patient quality of life. Efsubaglutide Alfa: Although not a traditional antibody, this GLP-1-based fusion protein exhibits exceptional efficacy and safety in type 2 diabetes management, making it a promising long-acting therapeutic option.
The successful approval of these therapies not only expands the arsenal of available treatments but also sets the stage for future advancements in drug development, providing invaluable technical insights and research breakthroughs for the pharmaceutical industry.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!