Genentech's Subcutaneous Ocrevus Nearly Halts Relapses and Brain Lesions in MS Over One Year
Roche subsidiary Genentech unveiled results from the Phase III OCARINA II trial examining Ocrevus (ocrelizumab), a research-based subcutaneous injection administered biannually for 10 minutes. The findings reveal a near-total reduction of clinical relapses and brain lesions in patients with relapsing or primary progressive multiple sclerosis, underscoring the promising advantages of this experimental formulation.
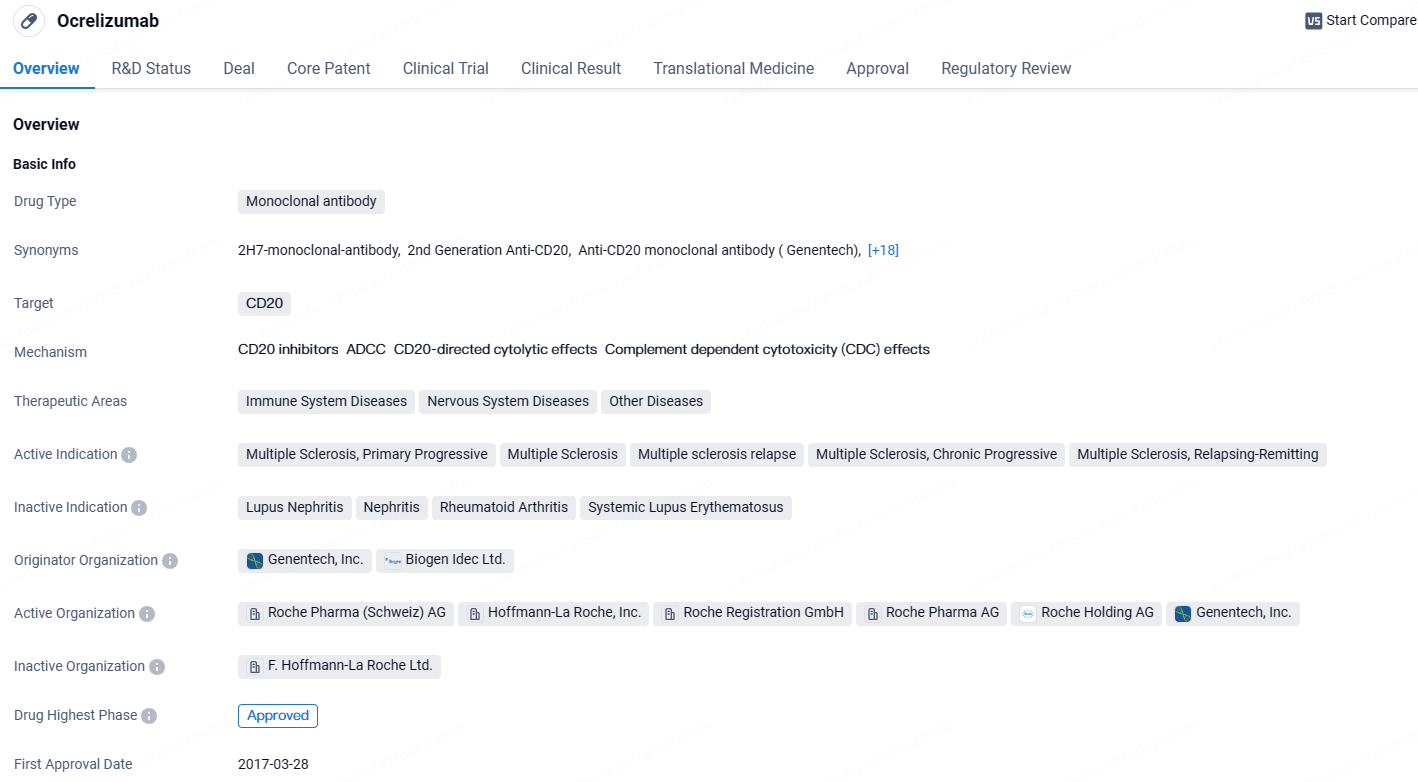
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Administration of subcutaneous Ocrevus resulted in quick and sustained elimination of B-cells in the bloodstream. This data is slated for an oral presentation at the 76th American Academy of Neurology Annual Meeting, scheduled for April 13-18 in Denver, and has earned the designation of a distinguished abstract by the AAN's scientific panel.
Subsequent findings with extended follow-up indicated that the subcutaneous administration of Ocrevus nearly completely inhibited both relapses and MRI evidence of disease activity through 48 weeks, showcasing an annualized relapse rate of 0.04. The majority of patients presented no T1 gadolinium-enhancing lesions and experienced no growth in T2 lesions. These MRI findings are indicative of ongoing inflammatory activity and the overall disease burden, respectively. Moreover, exploratory measures from patient-reported outcomes revealed significant satisfaction and convenience with the use of Ocrevus SC.
"Further data from the OCARINA II study reemphasizes the potential advantages of Ocrevus administered subcutaneously for patients suffering from relapsing and progressive types of MS," noted Scott Newsome, D.O., principal investigator from Johns Hopkins University School of Medicine. "This formulation allows for effective B-cell suppression and remarkable control over new inflammatory disease manifestations, suggesting that subcutaneous Ocrevus could tailor to the specific requirements of MS patients and medical providers."
The biannual, 10-minute subcutaneous injections could potentially make Ocrevus more accessible in treatment settings lacking IV capabilities or where IV resources are constrained. The findings from the Phase III OCARINA II trial have been submitted to global health regulators following the initial shareout at ECTRIMS-ACTRIMS in 2023. The submissions to both the European Medicines Agency and the U.S. Food and Drug Administration have been approved, with decision dates anticipated in mid-2024 for EMA and September 2024 for the FDA.
Worldwide, over 300,000 individuals with MS have received treatment with Ocrevus IV. The drug has regulatory approval in excess of 100 nations, covering various continents including North and South America, Asia, Europe, as well as in Australia and Switzerland.
Genentech is devoted to pioneering clinical studies aimed at enhancing the understanding of MS, diminishing the progression of disability in RMS and PPMS, and elevating the treatment experiences for those affected by MS. Currently, there are over 30 active Ocrevus clinical trials that are contributing to the expanded knowledge regarding MS and its progression.
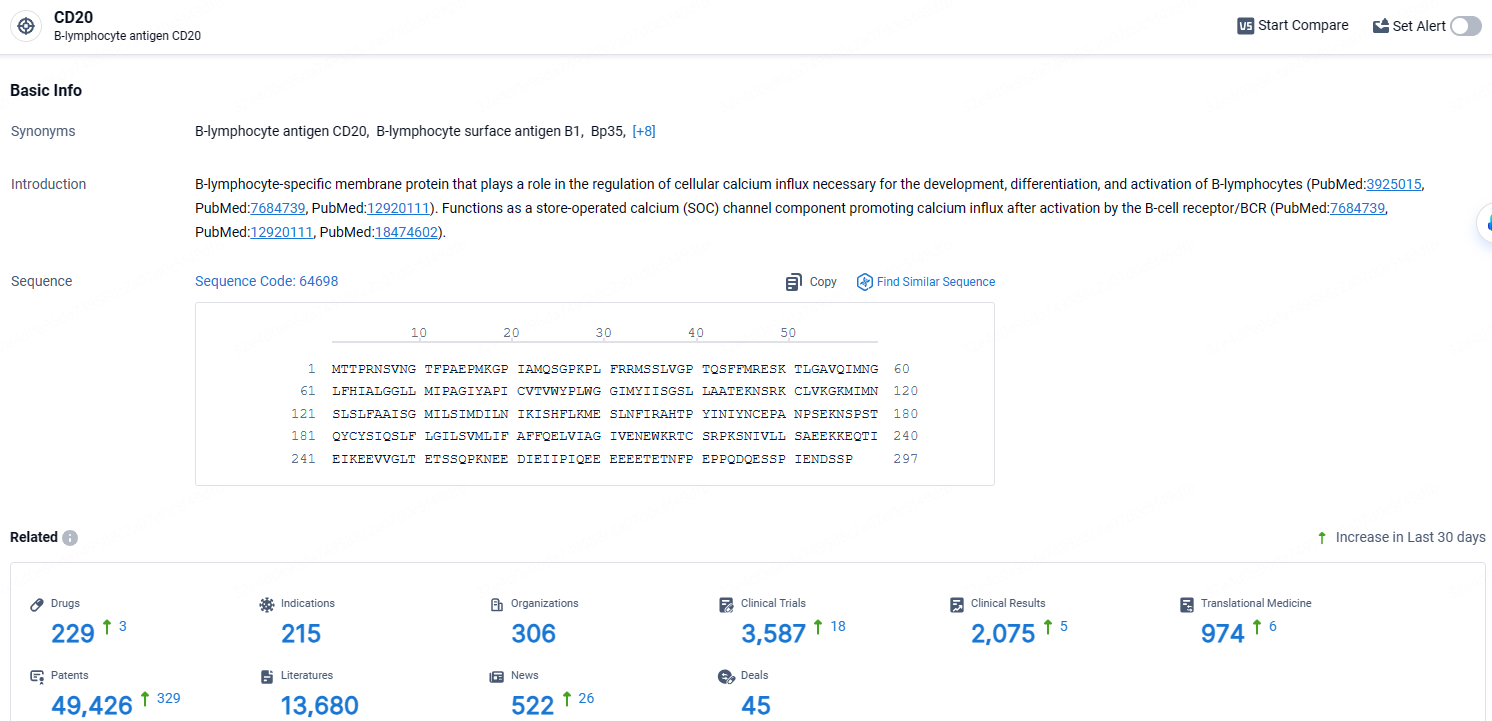
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 18, 2024, there are 229 investigational drugs for the CD20 target, including 215 indications, 306 R&D institutions involved, with related clinical trials reaching 3587, and as many as 974 patents.
crelizumab represents an important advancement in the field of biomedicine, particularly in the treatment of multiple sclerosis. Its approval in multiple forms of MS highlights its potential to address different aspects of the disease. As a monoclonal antibody targeting CD20, it offers a targeted approach to modulating the immune system and managing the symptoms of MS. The drug's fast track designation further underscores its potential to make a significant impact in the lives of patients with MS and potentially other diseases as well.