GPCR Family Anticancer Targets and Marketed Therapeutic Drugs (Part 3)
This article will continue to review the antitumor targets of the GPCR family and the therapeutic drugs that have been marketed.
05 SMO
The Smoothened (SMO) receptor is a key signal transduction receptor in the Hedgehog signaling pathway and belongs to the GPCR family, featuring the classical seven-transmembrane domain structure. The SMO receptor plays a vital role in the activation of the Hedgehog signaling pathway.
The discovery of the Hedgehog signaling pathway dates back to 1980 when Christiane Nüsslein-Volhard and Eric Wieschaus, during their research on the embryonic development of fruit flies, identified gene mutations affecting the segmental development of the Drosophila larvae through genetic screening. These genes were later named Hedgehog genes because their mutant flies' embryos had a spiky appearance reminiscent of hedgehogs.

The Hedgehog signaling pathway is highly conserved evolutionarily, playing significant roles not only in fruit flies but also in vertebrates, including in processes such as cell differentiation, cell proliferation, cellular senescence, tumorigenesis, malignant transformation, and tumor resistance. With further research, scientists have identified that the Hedgehog pathway involves a series of proteins, particularly core components that include two membrane proteins, Patched and Smoothened (SMO), along with three transcription factors (Gli1/2/3). The activity of the Hedgehog signaling pathway is regulated by multiple mechanisms, and abnormal pathway activity is associated with various diseases, including birth defects and tumors.
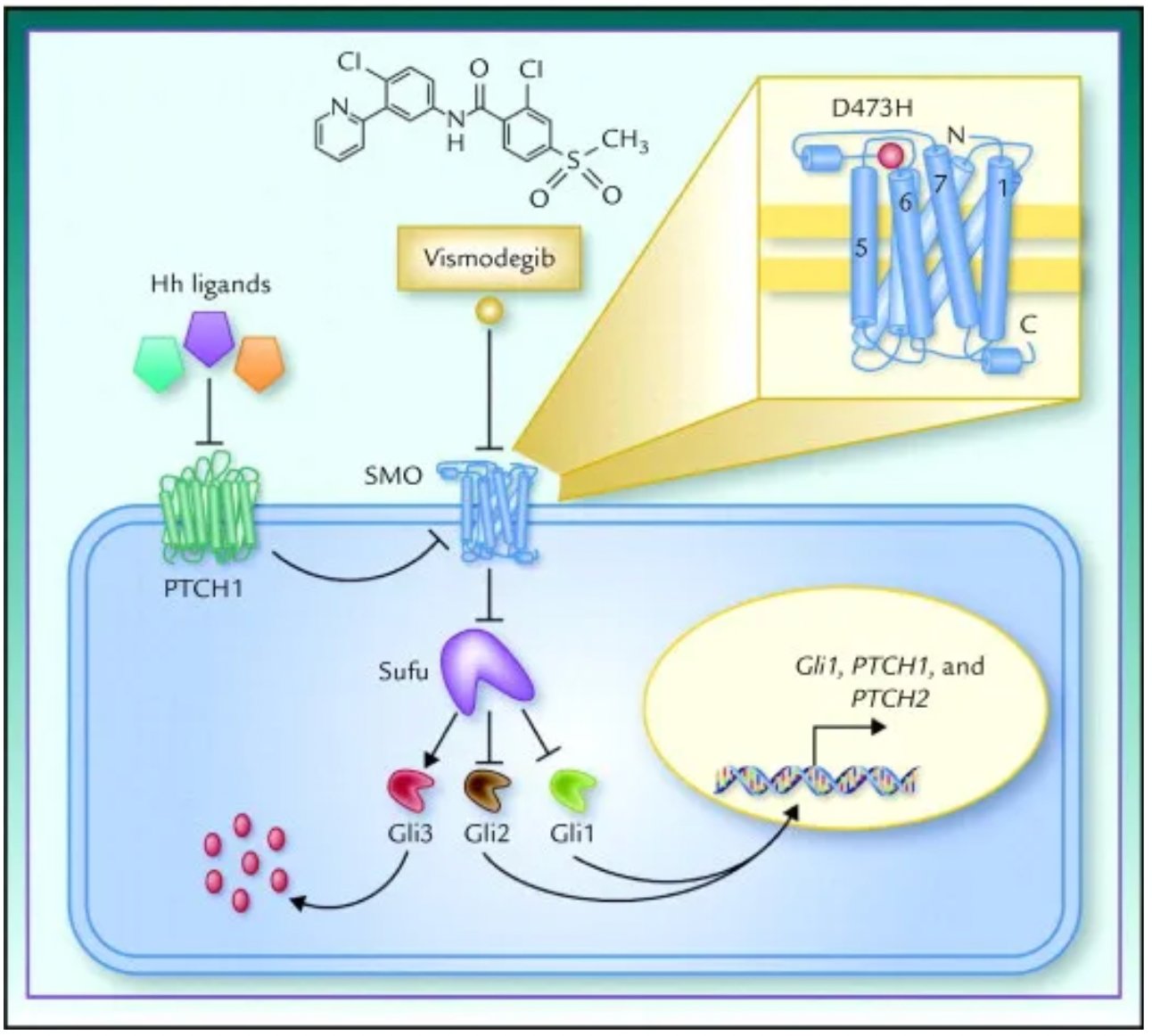
In the absence of a Hedgehog ligand, the SMO receptor is inhibited by the Patched (PTCH) receptor, which acts as a tumor suppressor protein that restricts SMO activity and prevents signal transduction. When Hedgehog ligands such as Sonic Hedgehog (Shh), Indian Hedgehog (Ihh), or Desert Hedgehog (Dhh) bind to the PTCH receptor, this inhibition is released, leading to the accumulation and activation of SMO within the cell.
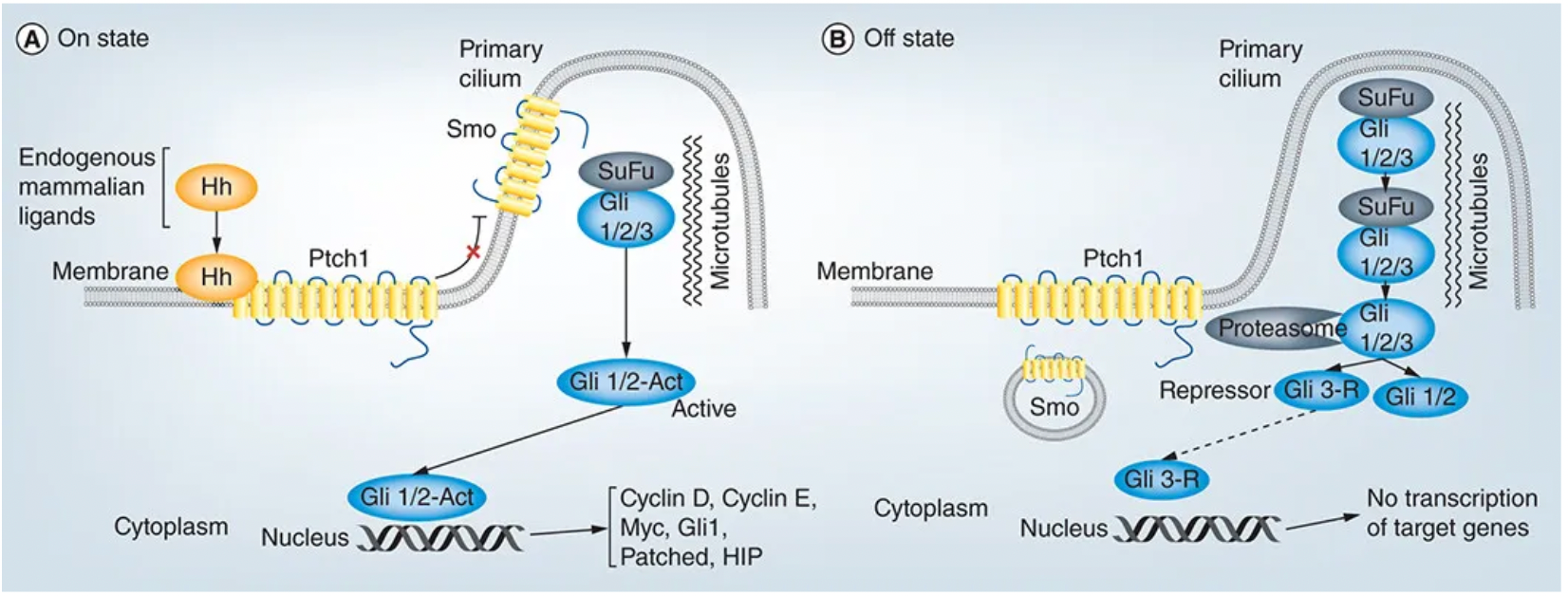
Activation of SMO further triggers downstream signaling pathways, particularly the activation of the GLI (Glioma-associated oncogene) family of transcription factors. These transcription factors exist in a repressive form when the signaling pathway is inactive, and upon SMO activation, they are dephosphorylated and converted into an active form, which then enters the nucleus to activate the expression of Hedgesten target genes.
Vismodegib (brand name Erivedge™), an oral SMO receptor antagonist, was developed by Genentech and approved by the FDA in January 2012 in the United States. It is primarily used for the treatment of locally advanced or metastatic basal cell carcinoma, especially in patients who are not suitable for surgery or radiation therapy.
In 2009, Genentech first disclosed the early drug discovery data of the SMO receptor antagonist candidate drug GDC-0449. Researchers used mouse embryonic fibroblasts harboring a luciferase reporter gene plasmid and performed high-throughput screening downstream of Gli binding sites. When the Hedgehog signaling pathway in these cells was stimulated, the activity of luciferase could be measured optically. Hedgehog pathway antagonists reduce the strength of this signal, leading researchers to obtain lead compounds. Subsequent SAR (Structure-Activity Relationship) studies yielded the candidate drug GDC-0449. At a low dose of 12.5 mg/kg, GDC-0449 was able to completely regress tumors in an orthotopic xenograft mouse model of medulloblastoma that entirely depended on the Hedgehog signaling pathway.
Between 2009 and 2012, the Genentech team published two clinical research papers consecutively in the New England Journal of Medicine, reporting the efficacy and safety of Vismodegib in treating advanced basal cell carcinoma.
The results showed that among 33 patients with metastatic basal cell carcinoma, the independently assessed response rate was 30%. Among 63 patients with locally advanced basal cell carcinoma, the independently assessed response rate was 43%, with 13 patients (21%) achieving a complete response. The median duration of response for both groups was 7.6 months.
In terms of safety, over 30% of patients experienced adverse reactions such as muscle spasms, hair loss, taste disturbances, weight loss, and fatigue. It was reported that 25% of patients experienced severe adverse reactions; 7 patients died due to adverse reactions.
Sonidegib (NVP-LDE225), developed by the Novartis team, is a SMO receptor antagonist also used for the treatment of locally advanced, recurrent basal cell carcinoma post-surgery and radiation, or in cases unsuitable for surgery and radiation. Sonidegib works by inhibiting a mechanism known as SMO activation, thereby blocking the Hh signaling pathway and exhibiting antitumor activity across various animal models. In a transgenic mouse pancreatic tumor model, tumors in mice treated with Sonidegib were reduced by 95% compared to untreated mice.
In 2014 and 2015, the Novartis team published clinical research papers detailing trial data for Sonidegib used in patients with advanced or metastatic basal cell carcinoma. In the primary efficacy analysis cohort, 20 out of 55 patients (36%) receiving 200 mg of Sonidegib achieved an objective response, and 39 out of 116 patients (34%) receiving 800 mg of Sonidegib achieved an objective response. Among the 200 mg Sonidegib group, 18 of 42 patients (43%) with locally advanced basal cell carcinoma achieved an objective response via central review, and 2 out of 13 patients (15%) with metastatic disease also achieved an objective response. In the 800 mg Sonidegib group, 35 out of 93 locally advanced patients (38%) obtained an objective response through central review, and 4 out of 23 metastatic disease patients (17%) also achieved an objective response.
Compared to the 800 mg group, the 200 mg group experienced fewer adverse events leading to dose reduction or treatment discontinuation. The most common grade 3-4 adverse events were elevated creatine kinase and increased lipase levels. In the 200 mg group of 79 patients, 11 (14%) experienced severe adverse events; in the 800 mg group of 150 patients, 45 (30%) experienced severe adverse events. Overall, the benefit-risk ratio of 200 mg Sonidegib provides a new treatment option for patients with advanced basal cell carcinoma.
In June 2015, the U.S. FDA approved Sonidegib for the treatment of adult basal cell carcinoma. In 2021, Sonidegib was approved for market in Mainland China, becoming the first SMO receptor antagonist approved in China.
Following the market entries of Genentech's Vismodegib and Novartis's Sonidegib Phosphate, Pfizer's SMO antagonist drug, Glasdegib Maleate, was approved in 2018 for the treatment of patients with acute myeloid leukemia.
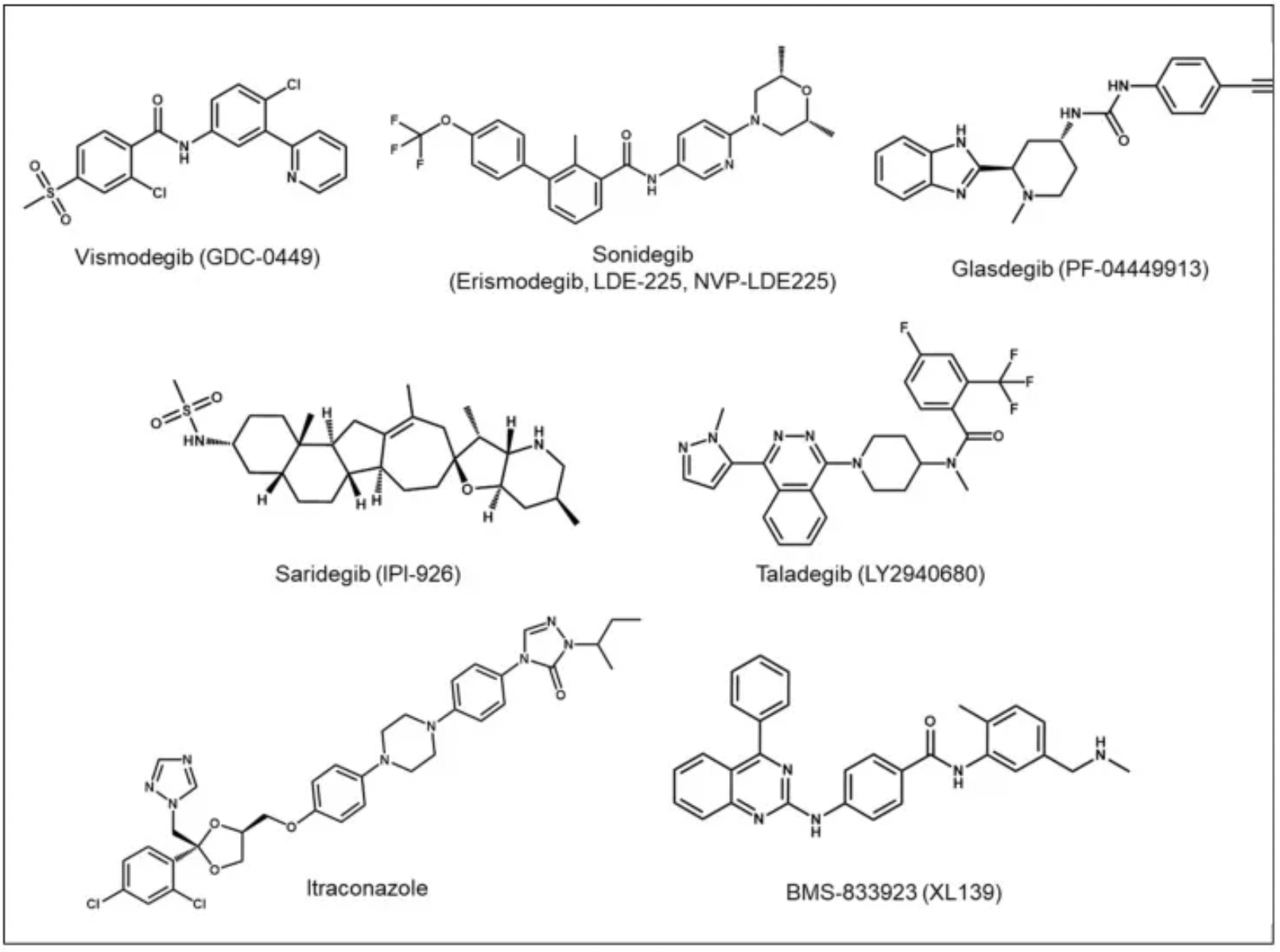
The representative molecular formula for SMO antagonists is as follows:
06 TSHR
The Thyroid-Stimulating Hormone Receptor (TSHR) is a gene that encodes a GPCR controlling the metabolism of thyroid cells. This protein acts as a receptor for thyroid-stimulating hormone (TSH) and thyroxine. In addition to the thyroid, TSHR expression has also been confirmed in various tissues including the brain, bone marrow, peripheral blood, and bones.
Thyroid-stimulating hormone (TSH) is a pituitary hormone that stimulates the production of thyroid hormones by the thyroid gland. TSH is primarily synthesized in the thyrotroph cells of the anterior and nodular portions of the pituitary gland. Additionally, hematopoietic cells in the bone marrow, particularly monocyte/macrophage precursors, have also been shown to be a source of TSH. TSH binds to TSHR in the thyroid, stimulating the production and release of thyroid hormones.
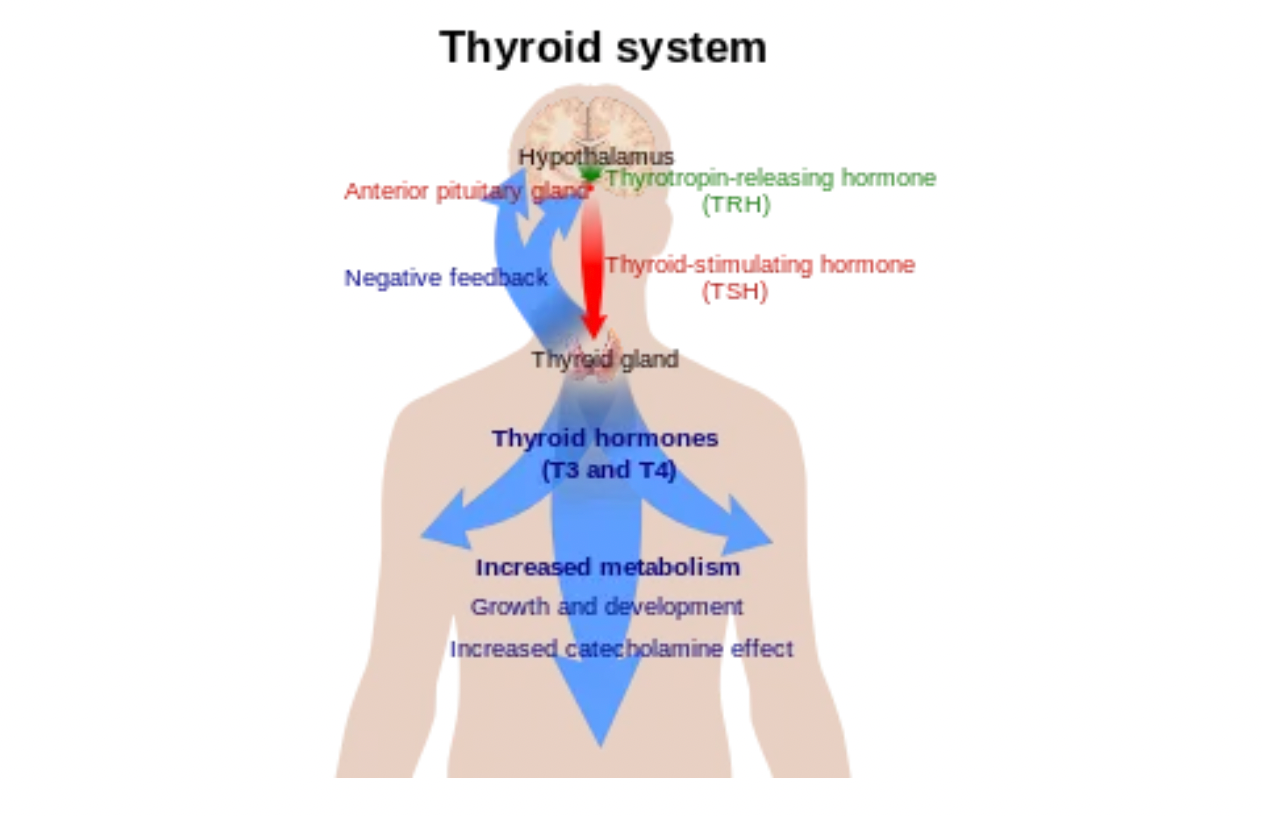
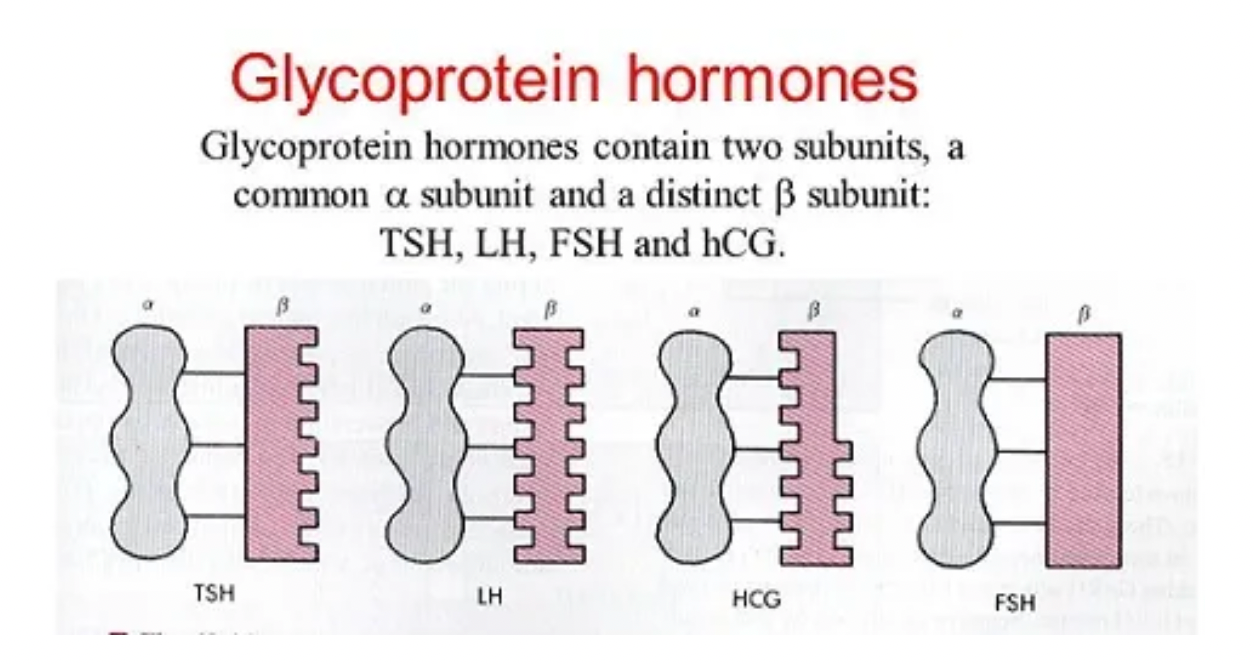
TSH is a non-covalently linked heterodimeric glycoprotein composed of alpha and beta subunits. The alpha subunit is common with other glycoprotein hormones, such as luteinizing hormone and follicle-stimulating hormone, while the beta subunit is unique to each hormone.
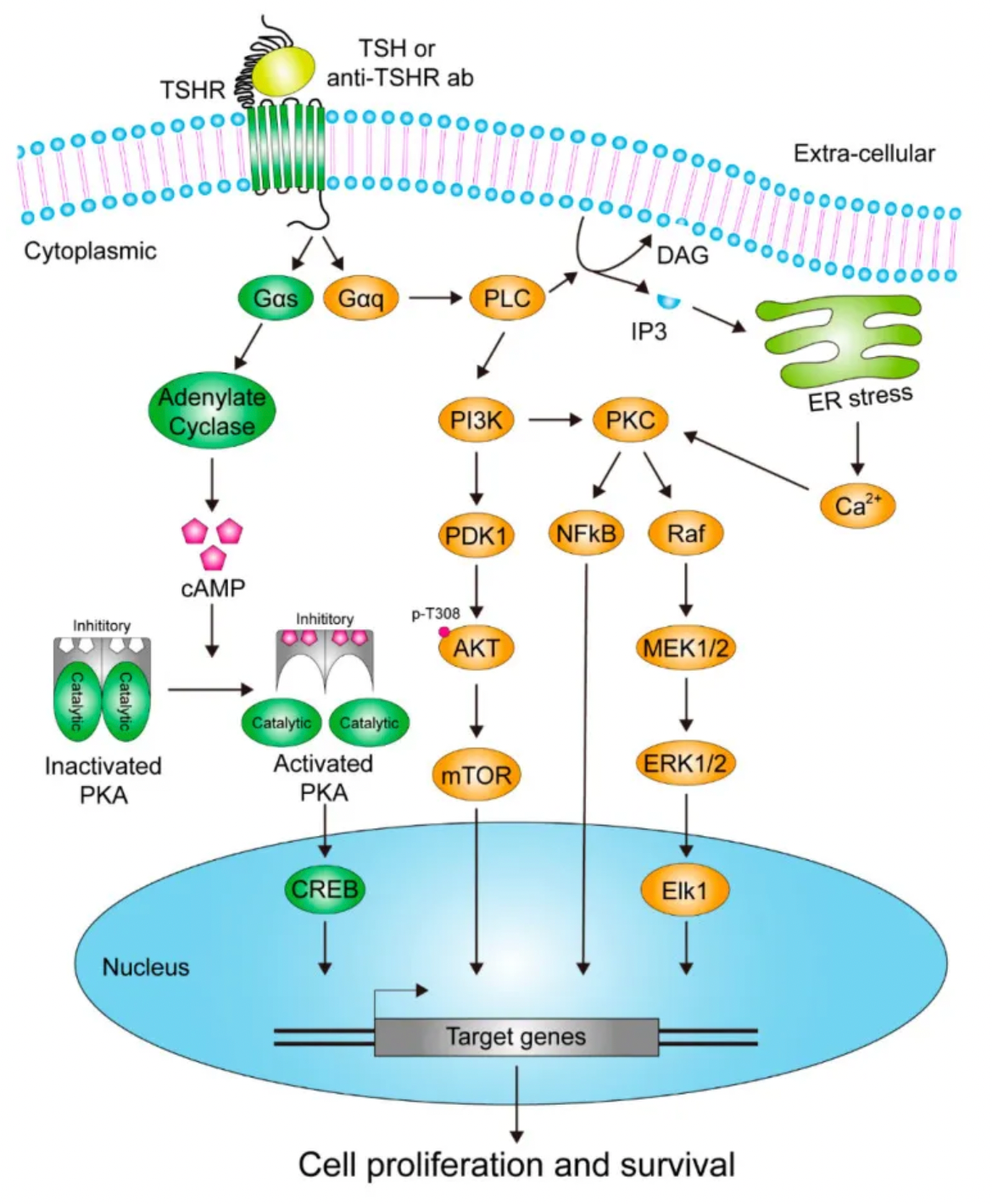
TSH signaling can stimulate the secretion of vascular endothelial growth factor (VEGF), leading to angiogenesis and accelerating genomic instability in thyroid cancer. The tumorigenic role of TSH in thyroid cancer has been established. Many patients with differentiated thyroid cancer undergo radioactive iodine ablation postoperatively to remove residual normal thyroid tissue. Several non-randomized studies suggest that radioactive iodine ablation can reduce mortality and recurrence rates.
Thyrotropin alfa is a recombinant form of thyroid-stimulating hormone, developed by Genzyme, and was initially approved by the U.S. FDA in 1998 as a diagnostic tool for managing patients undergoing recurrence monitoring of well-differentiated thyroid cancer. This product helps to enhance the sensitivity of tests while allowing patients to avoid potential debilitating symptoms associated with thyroid hormone withdrawal. In 2008, the FDA approved a new supplementary indication for Thyrotropin Alfa for use in conjunction with radioactive iodine to ablate or destroy residual thyroid tissue in patients who have undergone thyroidectomy for cancer.
A study published in the New England Journal of Medicine in 2012 demonstrated that low-dose radioactive iodine combined with thyrotropin alfa is as effective as high-dose radioactive iodine, with a lower incidence of adverse events. In the diagnosis and treatment of thyroid cancer, the use of Thyrotropin alfa enhances the uptake of radioactive iodine by these cells, thereby improving the effectiveness of radioactive iodine treatment or enhancing the clarity of imaging during radioactive iodine scans, helping physicians to more accurately assess the status of the disease.
Outside of thyroid cancer, the incidence of prostate cancer, lung cancer, and colorectal cancer has been linked to low levels of TSH. For example, various mutations such as missense mutations, nonsense mutations, silent mutations, frameshift deletions, and in-frame deletions have been observed in cancers like colorectal cancer, skin cancer, and thyroid cancer.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!