GPCR Family Anticancer Targets and Marketed Therapeutic Drugs (Part 4)
This article will continue to review the antitumor targets of the GPCR family and the therapeutic drugs that have been marketed.
07 GPRC5D
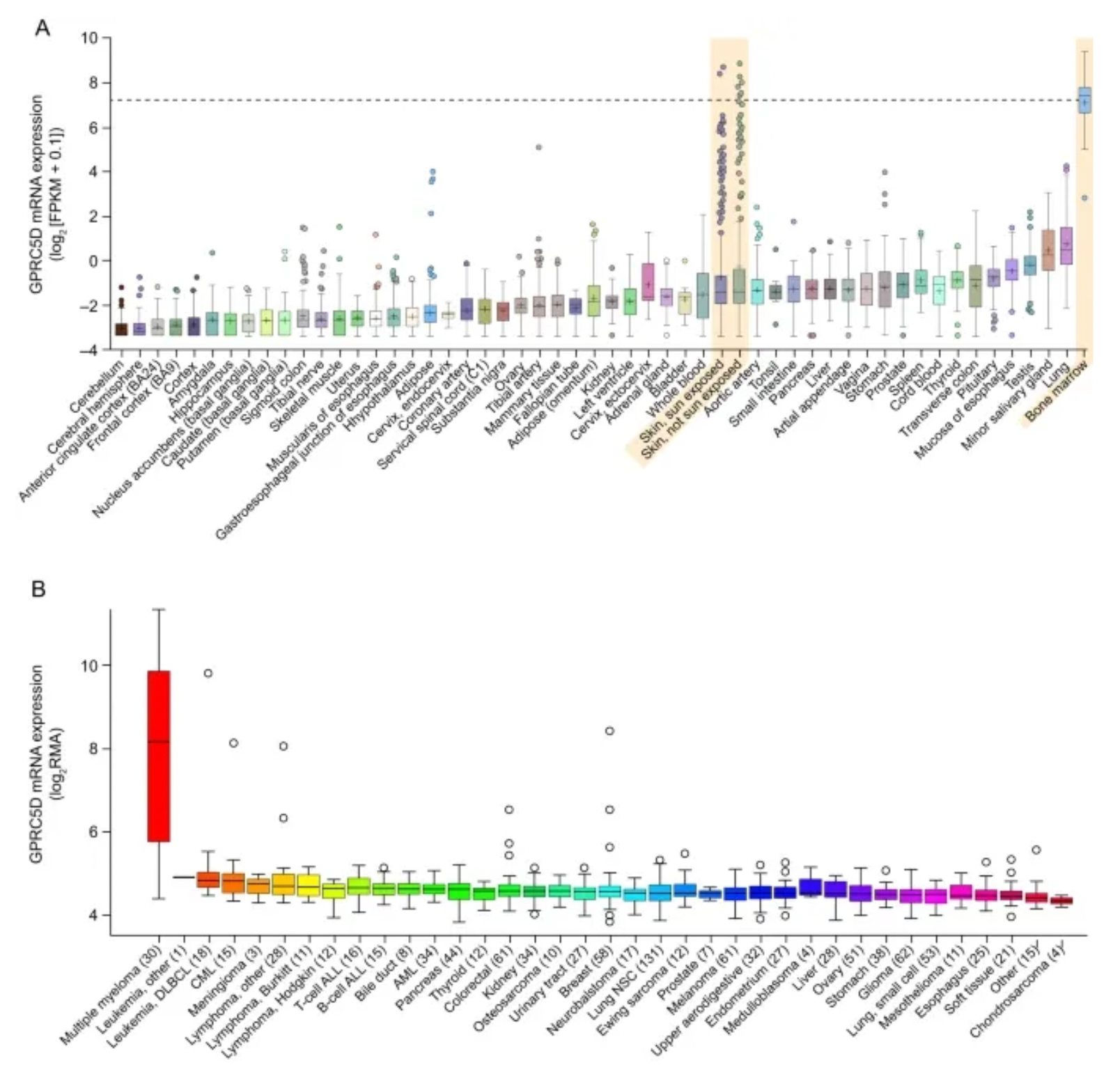
GPRC5D is a Class C type-7 transmembrane GPCR receptor protein, primarily expressed in cells with a plasma cell phenotype, including the majority of malignant plasma cells from patients with multiple myeloma (MM). GPRC5D is an orphan receptor, whose ligand and signaling mechanisms have yet to be identified. In patients with multiple myeloma, the expression levels of GPRC5D messenger RNA correlate with the plasma cell burden and genetic abnormalities such as Rb-1 deletion and high-risk MM. Compared to normal cells, GPRC5D mRNA levels are higher in myeloma plasma cells. The selective expression of GPRC5D suggests that it could be a potential target for effector cell-mediated therapy in treating plasma cell disorders like MM.
The accompanying figure shows the expression differences of GPRC5A in normal tissues versus various tumor tissues.
Talquetamab is a full-length, humanized IgG4 bispecific antibody developed by Janssen Pharmaceuticals that targets GPRC5D and the CD3 receptor. It is the first GPRC5D-targeting agent approved for the treatment of triple-exposed relapsed/refractory multiple myeloma patients and was approved for use in the United States in August 2023. Bispecific antibodies are designed to overcome some of the limitations of traditional monoclonal antibodies and enhance therapeutic efficacy. T-cell-engaging bispecific antibodies have two antigen-binding sites: one for CD3 on the surface of T-cells and another for a target antigen expressed on tumor cells. The simultaneous binding to T-cell surface receptors and tumor-specific antigens brings T cells into proximity with tumor cells, leading to synapse formation, activation of T cells, and subsequent killing of malignant cells. T-cell-engaged antibodies may overcome certain forms of tumor-mediated immune evasion since they do not require antigens to be presented by major histocompatibility complex molecules, which are often downregulated or lost in tumor cells, to perform their effector functions.
08 GHSR
Ghrelin is a small peptide consisting of 28 amino acids, identified as a ligand for the growth hormone secretagogue receptor (GHSR). Beyond its original function in stimulating the release of growth hormone from the pituitary, ghrelin has various roles, including regulating energy balance, gastric acid secretion, appetite, insulin secretion, gastrointestinal motility, and the renewal of gastric and intestinal mucosa.
Cachexia is a hypercatabolic state characterized by accelerated loss of skeletal muscle in the presence of chronic inflammatory response, seen in conditions such as advanced cancer, chronic infections, AIDS, heart failure, rheumatoid arthritis, and chronic obstructive pulmonary disease. Cachexia is prevalent in various malignancies, affecting nearly half of patients with advanced cancer. Although cancer patients commonly experience decreased appetite and weight loss, the significant weight loss in cachexia cannot be solely attributed to inadequate caloric intake. Insufficient oral intake combined with complex metabolic abnormalities can lead to increased basal energy expenditure, ultimately resulting in the reduction of lean body mass due to skeletal muscle consumption. Unlike simple starvation, where caloric deficiency can be reversed by appropriate feeding, weight loss in cachexia cannot be adequately treated through aggressive nutritional support.
Anamorelin, developed by Helsinn Healthcare SA, is an orally administered non-peptidic drug, a selective agonist for the growth hormone secretagogue receptor (GHSR). It has effects on promoting appetite and anabolic metabolism, primarily used in treating cancer cachexia and anorexia. The drug was approved for market in Japan in 2021.
Anamorelin acts by stimulating the levels of growth hormone (GH), insulin-like growth factor 1 (IGF-1), and insulin-like growth factor-binding protein 3 (IGFBP-3) in the human body, without affecting the plasma levels of other hormones such as prolactin, cortisol, insulin, glucose, adrenocorticotropic hormone, luteinizing hormone, follicle-stimulating hormone, or thyroid-stimulating hormone. It is capable of increasing appetite, overall body weight, lean body mass, and muscle strength.
However, on May 18, 2017, the European Medicines Agency (EMA) recommended the refusal of the marketing authorization for the drug, because although Anamorelin had a slight effect on lean body weight in patients, it showed no proven effect on handgrip strength or patient quality of life. Additionally, following inspections of clinical research facilities, the agency considered that the safety data of the drug were not adequately documented.
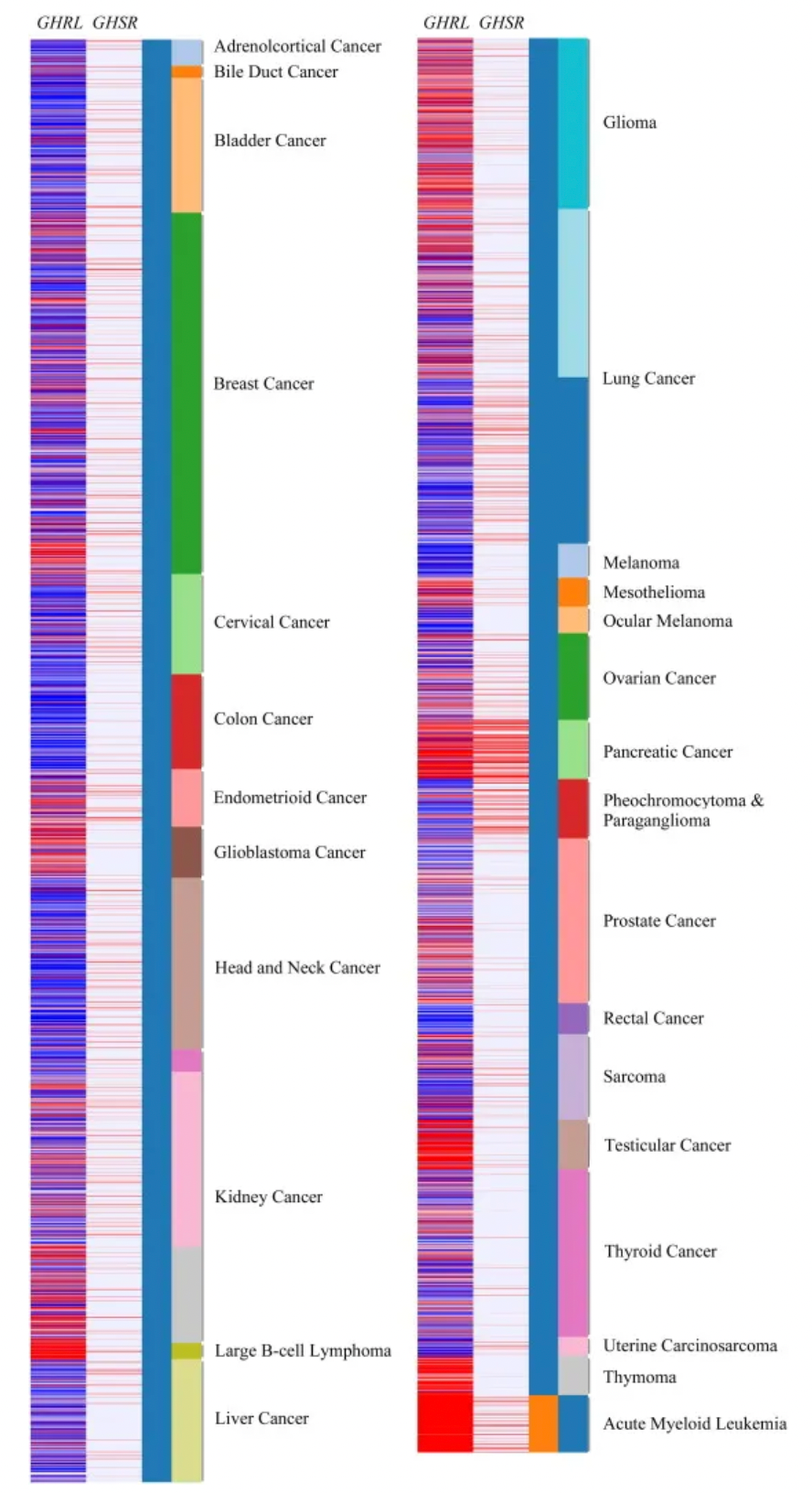
Furthermore, increasing evidence also reveals ghrelin's role in regulating several processes related to cancer progression, especially regarding metastasis and proliferation. Transcriptomic data generated by RNA sequencing and matched clinical data from large cancer cohorts in The Cancer Genome Atlas (TCGA) indicate elevated expressions of GHRL and GHSR in various cancers including cholangiocarcinoma, breast cancer, endometrial carcinoma, glioblastoma, renal cancer, diffuse large B-cell lymphoma, glioma, lung cancer, mesothelioma, pancreatic cancer, prostate cancer, sarcoma, testicular cancer, thymoma, and acute myeloid leukemia. This suggests a pathological role for these genes in cancer, making the development of selective antagonists to inhibit GHSR activity a potential anti-tumor strategy. Consequently, targeted anti-tumor research on GHSR undoubtedly requires further mechanistic studies and exploration.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!