GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (1)
This article will introduce the GPCR targets and pipelines that received attention for Novartis, Bristol Myers Squibb, Amgen, Bayer, and Gilead at the JP Morgan Healthcare Conference 2024. For more details, please follow the link below.
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (2)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (3)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (4)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (5)
Novartis in JPM 2024
Novartis's differentiated characteristics provide an extremely attractive opportunity for creating value for shareholders: it is an innovation-driven pharmaceutical company with 4 core therapeutic areas and 2+3 technology platforms.

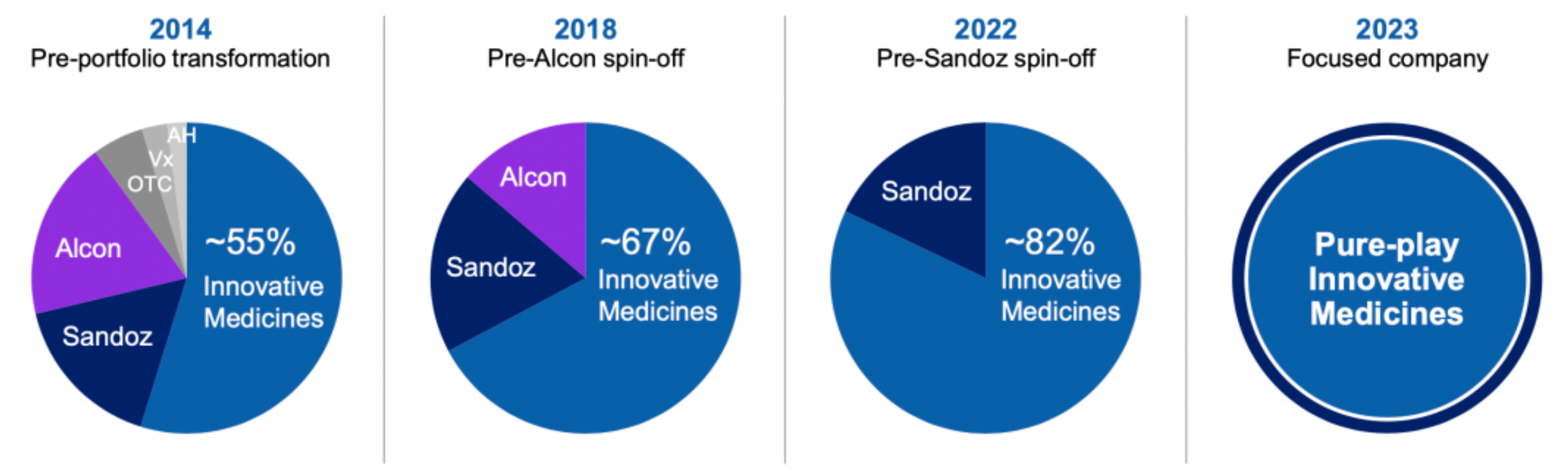
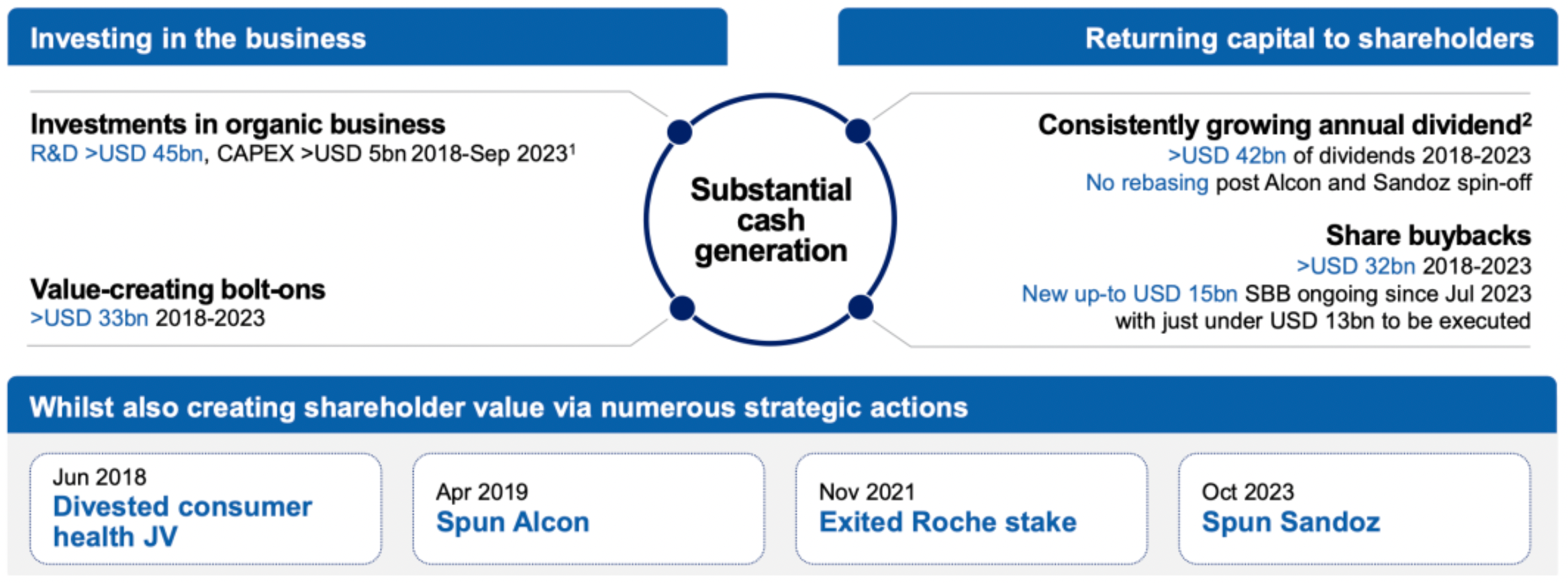
Over the past year, Novartis has signed more than 15 strategic deals totaling over $6 billion in order to bolster our product portfolio within key therapeutic areas and technology platforms.

Promoting three breakthrough technologies to unlock significant mid- to long-term growth potential for Novartis.
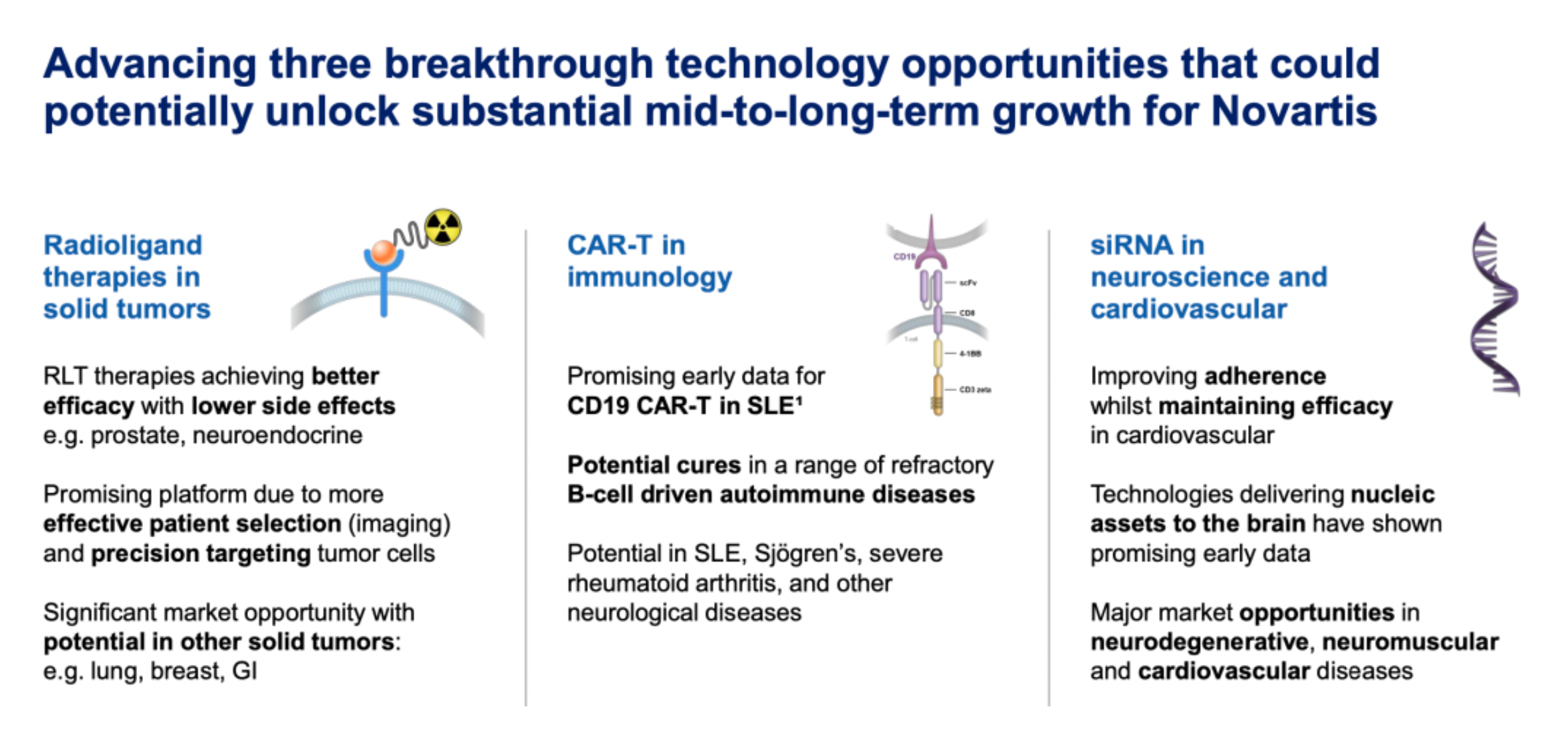
Entresto (Sacubitril/Valsartan) achieved an annualized sales figure of $5.9 billion in the third quarter, with a 31% increase in Q3: Sacubitril is a neprilysin inhibitor, which as a single agent has not yet been approved for marketing; Valsartan is an AT1R receptor antagonist. The combination formulation of Sacubitril and Valsartan, developed by Novartis, is used to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure and reduced ejection fraction.
Atrasentan is currently undergoing a phase 3 clinical study with clinically meaningful primary endpoints: Atrasentan is an ETA receptor antagonist, currently in phase 3 clinical trials for the treatment of immunoglobulin A (IgA) nephropathy.
Lutathera (Lutetium Dotatate LU-177) ongoing phase 3 (NETTER-2) results show the potential of radioligand therapy in early-stage gastroenteropancreatic neuroendocrine tumors (GEP-NETs): Lutathera is a radiopharmaceutical consisting of 177 Lu-labeled somatostatin analog, with the mechanism of action being an SSTR2 receptor antagonist. Lutathera features a high uptake in tumors with a prolonged retention time; biodistribution-wise, Lutathera has a lower systemic retention, resulting in reduced toxicity and associated risks.
Bristol Myers Squibb in JPM 2024
Bristol Myers Squibb is writing a new chapter in its history, entering another period of innovation, and will continue to provide patients with innovative medications.
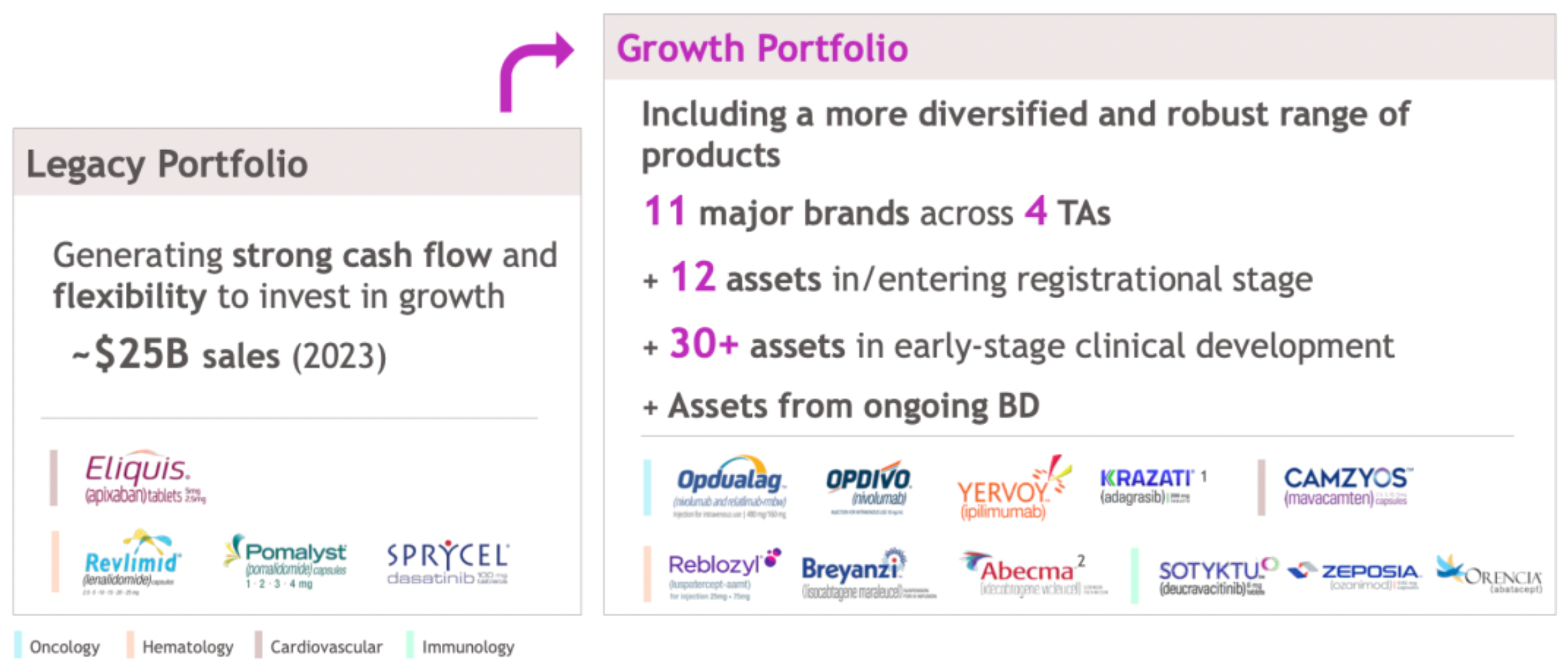
In 2023, the company has expanded its product portfolio, which has become more diverse and robust, comprising 11 major brands spanning across four key therapeutic areas. The company has 12 assets at or entering the registration phase, with over 30 assets in early-stage clinical development.
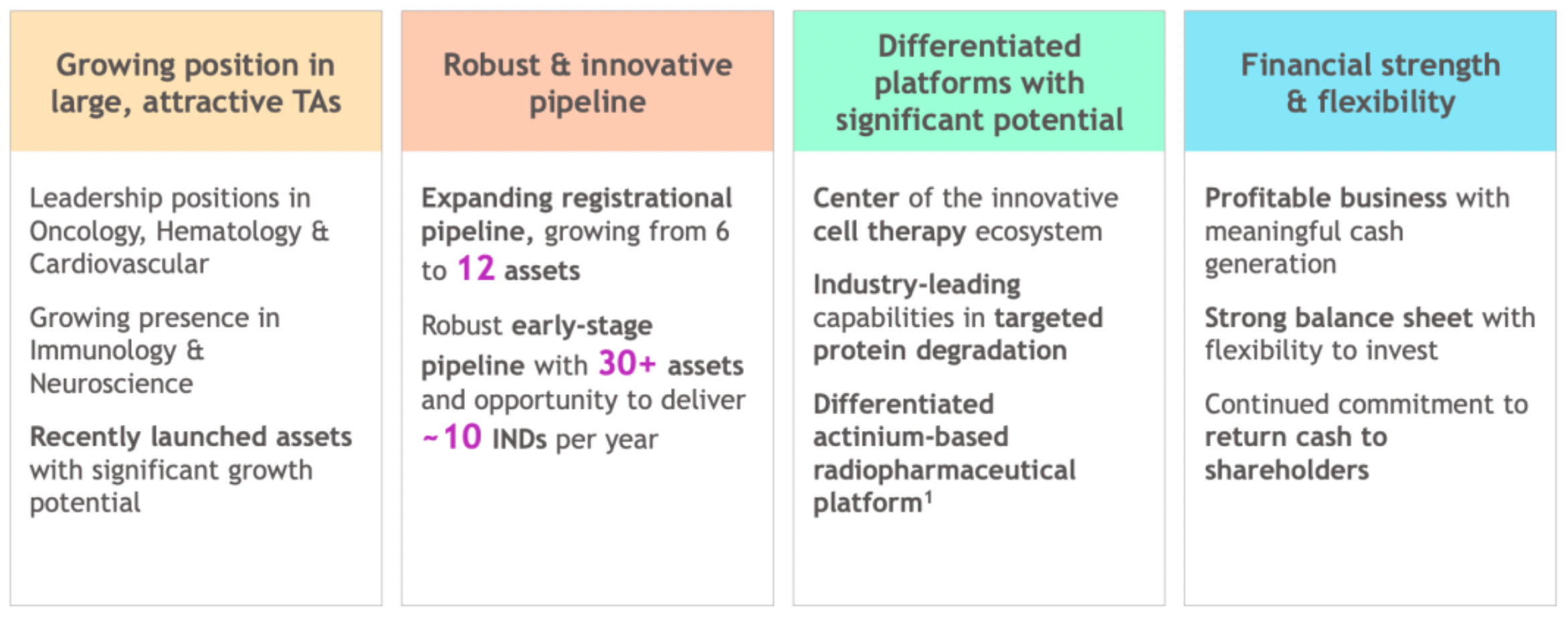
In three primary directions, consolidate the company's leading position.

In the fields of immunology and neuroscience, influence continues to expand.
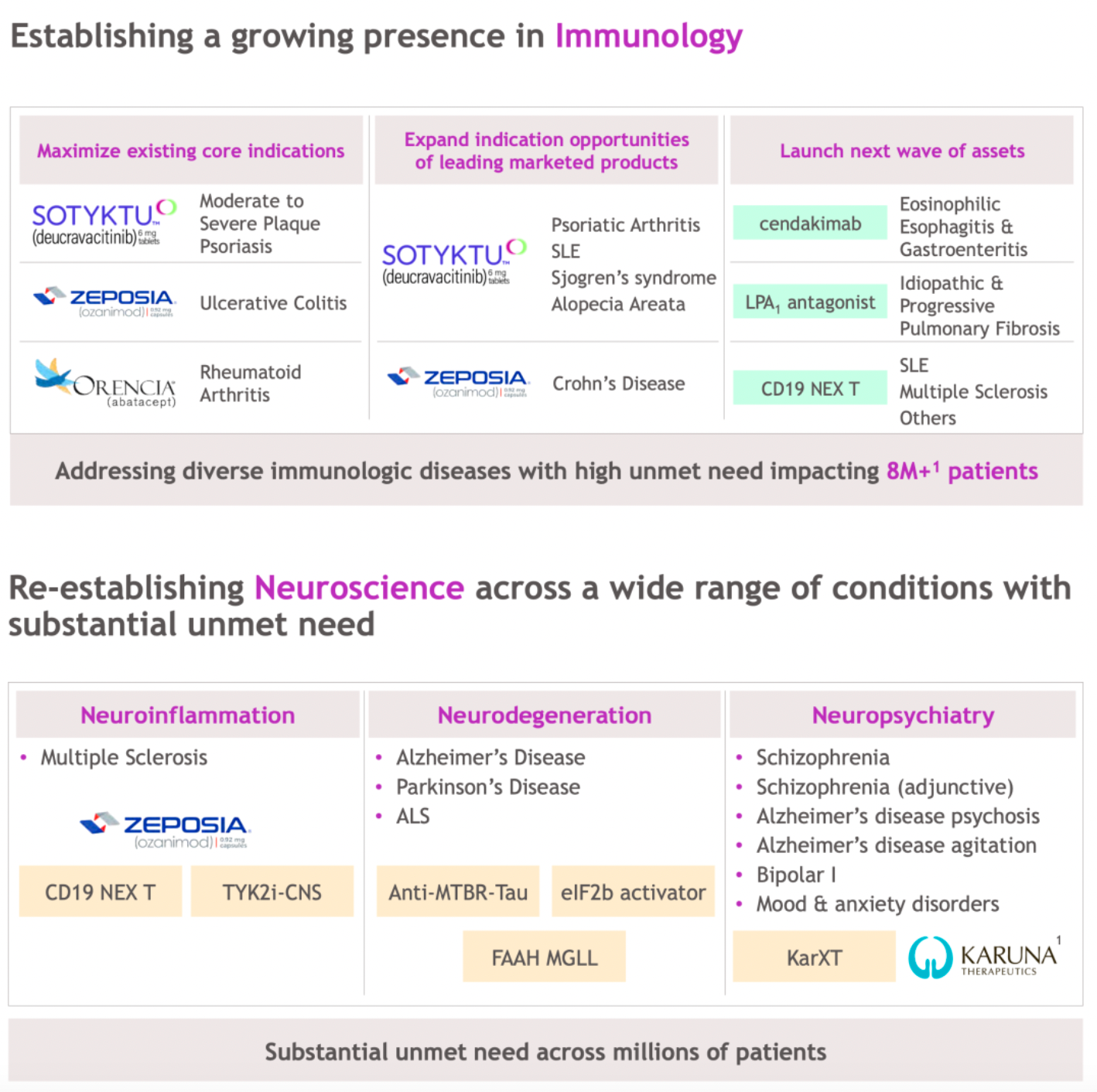
BMS-986278 is a LPA1 receptor antagonist currently in Phase 3 clinical development for the treatment of idiopathic pulmonary fibrosis or progressive pulmonary fibrosis. In addition, two other LPA1 receptor antagonists from the company, 18F-BMS-986327 and BMS-986337, are in Phase 1 clinical trials.
Both GPRC5D CAR-T and dual-target BCMAxGPRC5D CAR-T products target the Class C GPCR member GPRC5D. In August 2023, a dual-target GPRC5DxCD3 bispecific antibody developed by Johnson & Johnson was approved for marketing, making GPRC5D a popular research target.
BMS-986340 is a monoclonal antibody against CCR8, intended for the treatment of solid tumors and currently in Phase 1 clinical research.
Amgen in JPM 2024
Amgen is using a range of novel therapeutics to treat a variety of severe illnesses, including common conditions such as obesity, heart disease, migraines, chronic kidney disease, as well as rare diseases, cancer, and immune disorders.
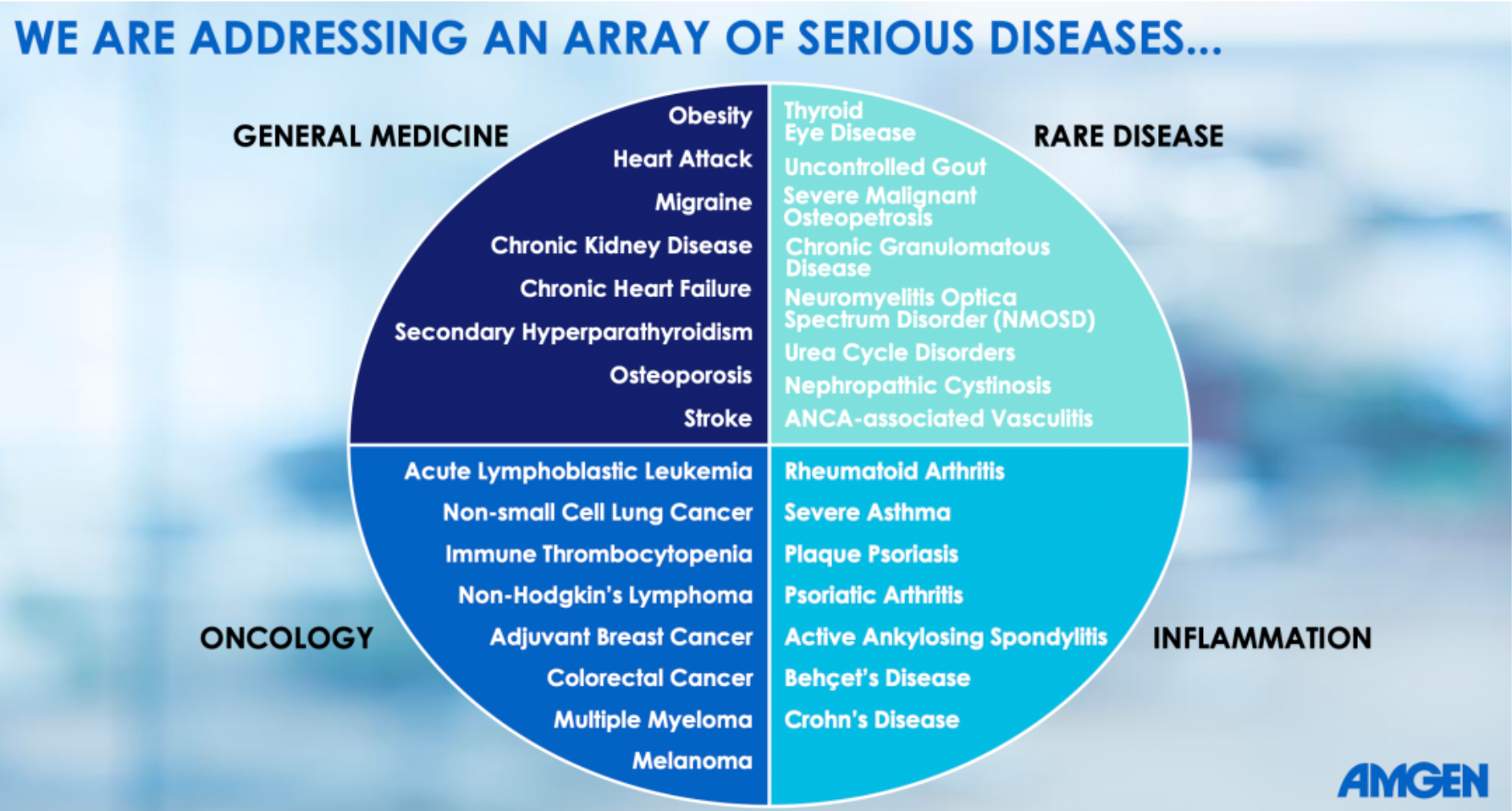

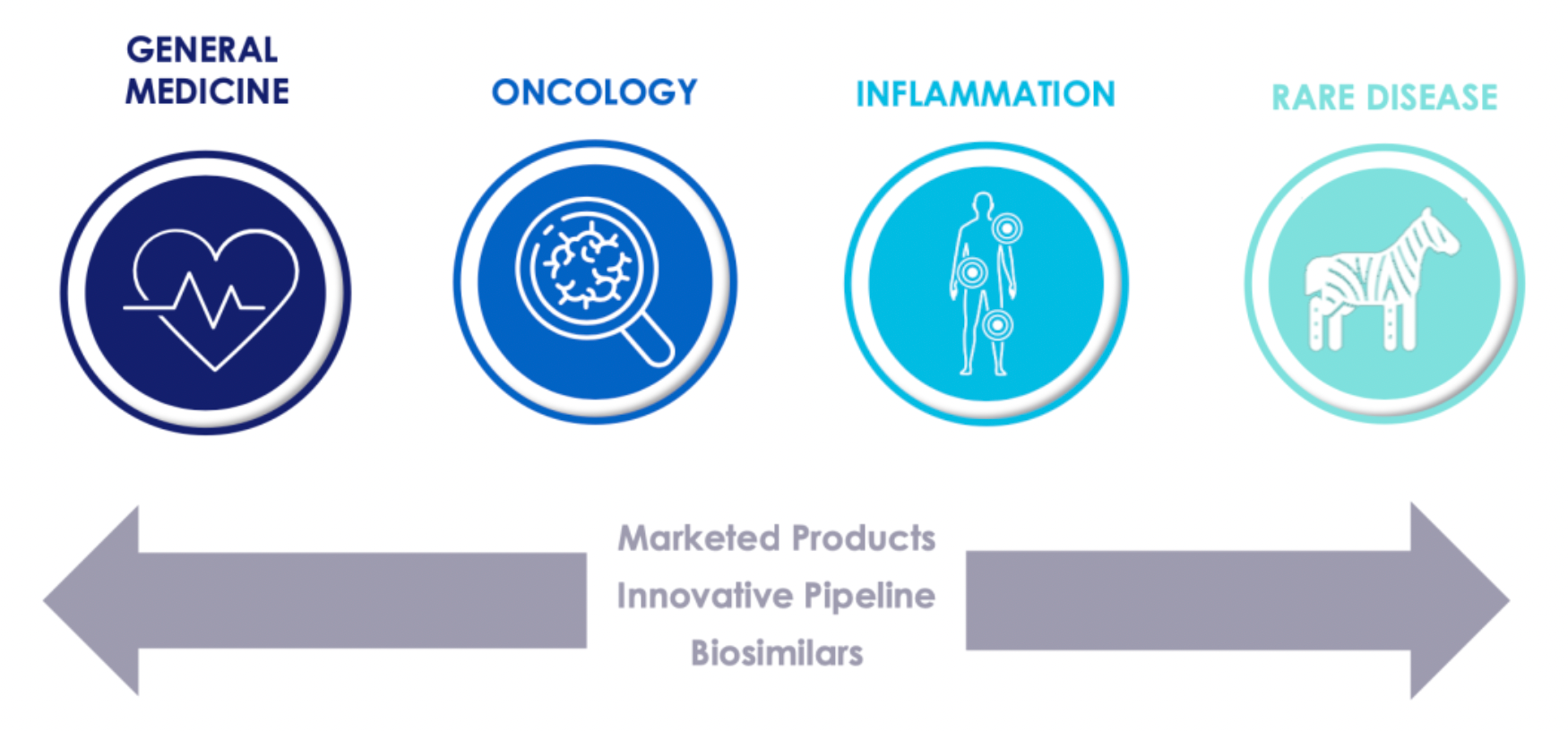
The Four Pillars of Promoting Long-Term Growth.
The pipeline projects for which the company is expected to achieve significant milestones in 2024.
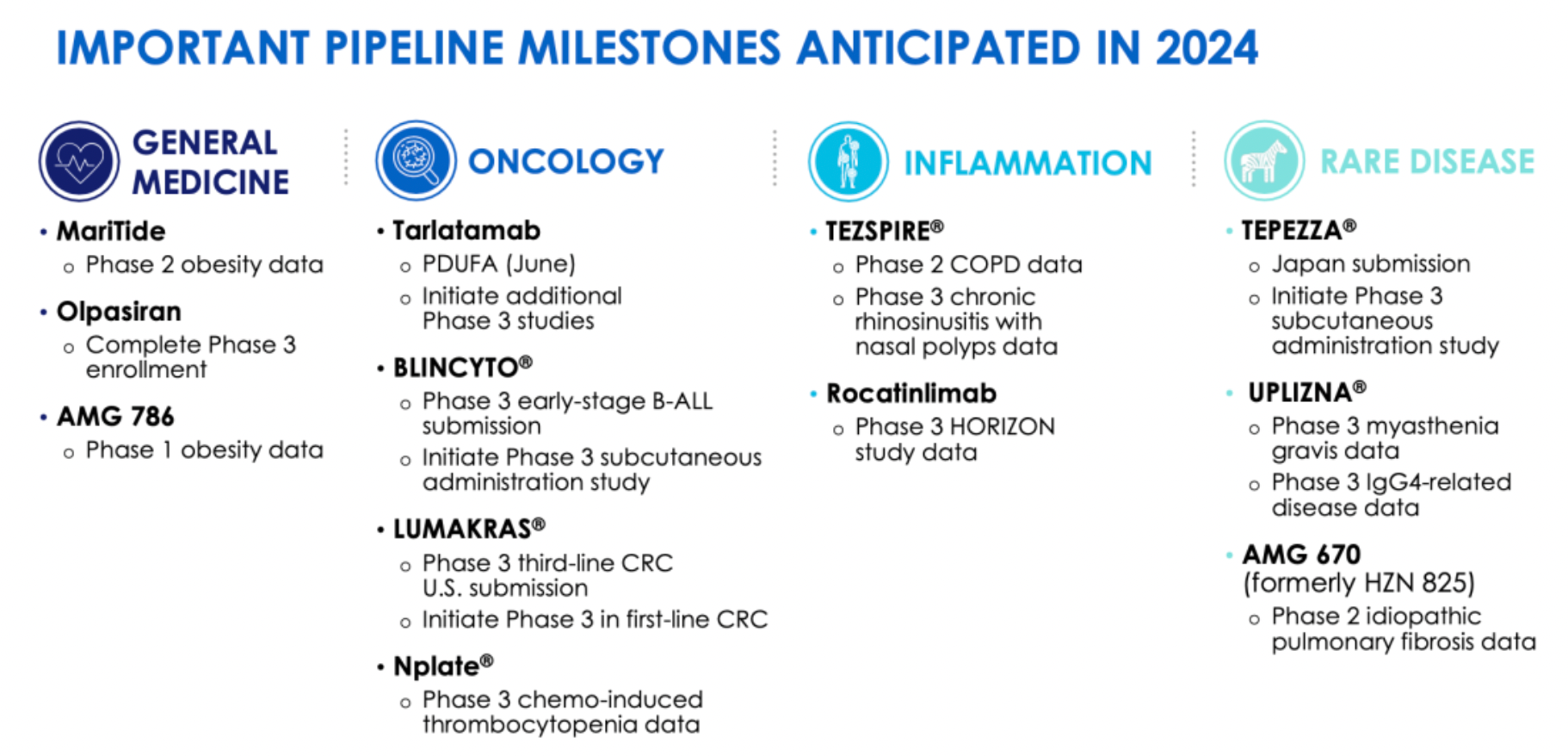
MariTide (maridebart cafraglutide) is a dual-specificity molecule currently undergoing Phase II clinical trials, which acts as a GIPR antagonist and a GLP-1R agonist. In addition, AMGEN's small molecule candidate drug AMG 786 (target not disclosed) is in Phase I clinical study. AMGEN hopes that the combined use of these two interventions will allow for quicker and more effective weight loss, as well as better weight maintenance.
Bayer in JPM 2024
Bayer continues to drive the sales of a multitude of medications, maximizing the full commercial value of its existing product portfolio while persistently redirecting resources towards brands and assets with high innovative potential.
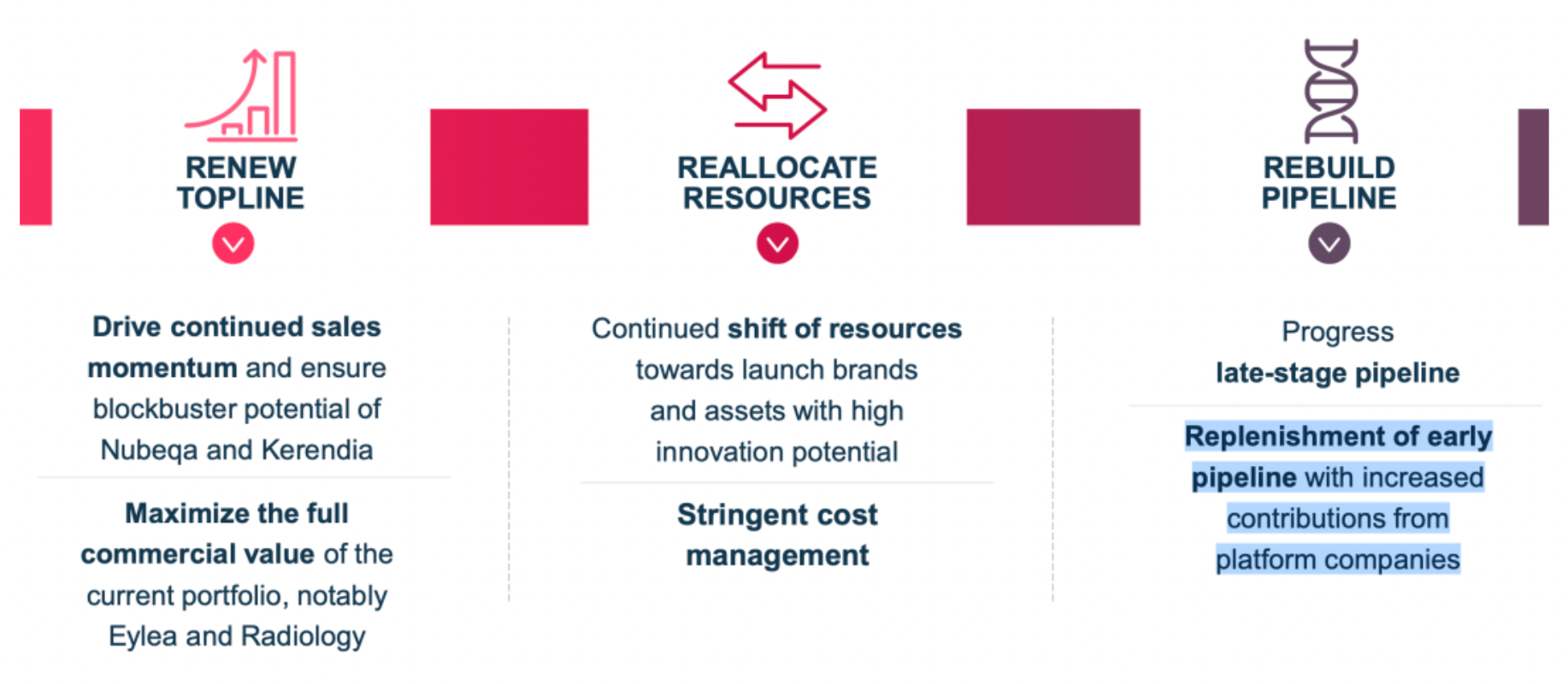
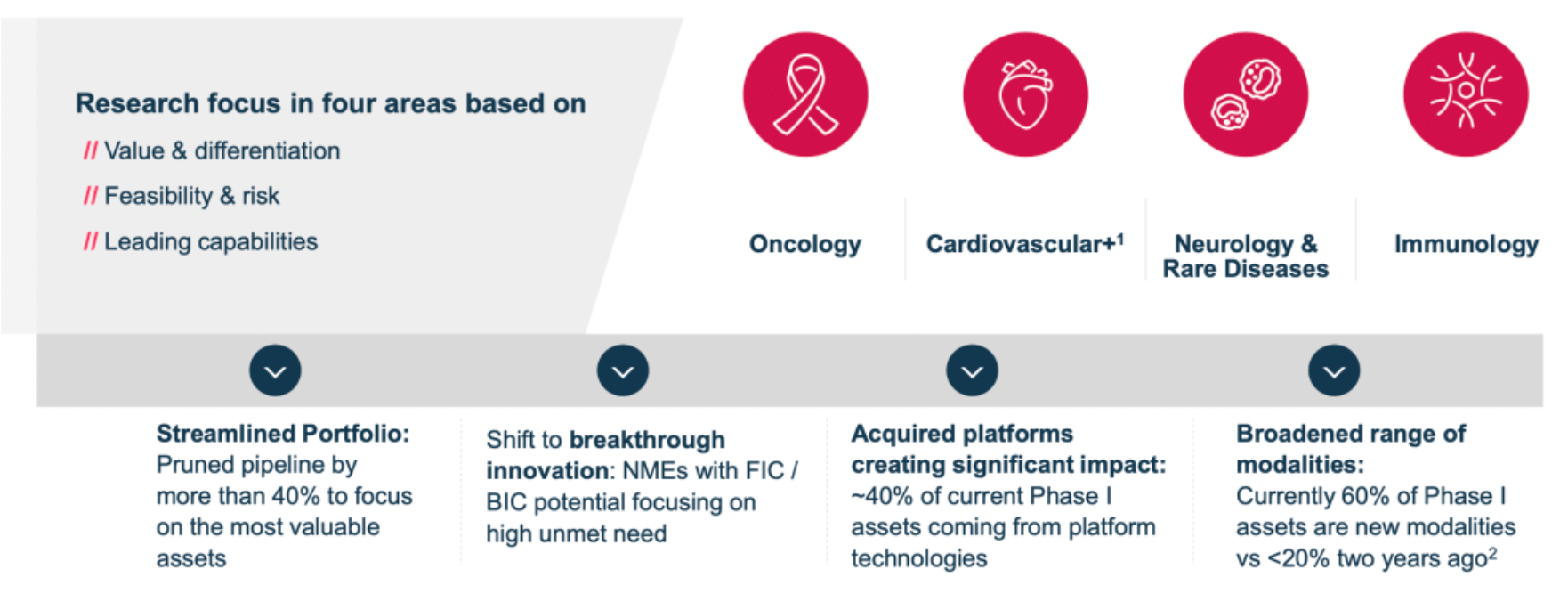
Focusing on four primary areas, which include oncology, cardiovascular, neurological and rare diseases, as well as immunological disorders.
Several pipelines will reach multiple important milestones.
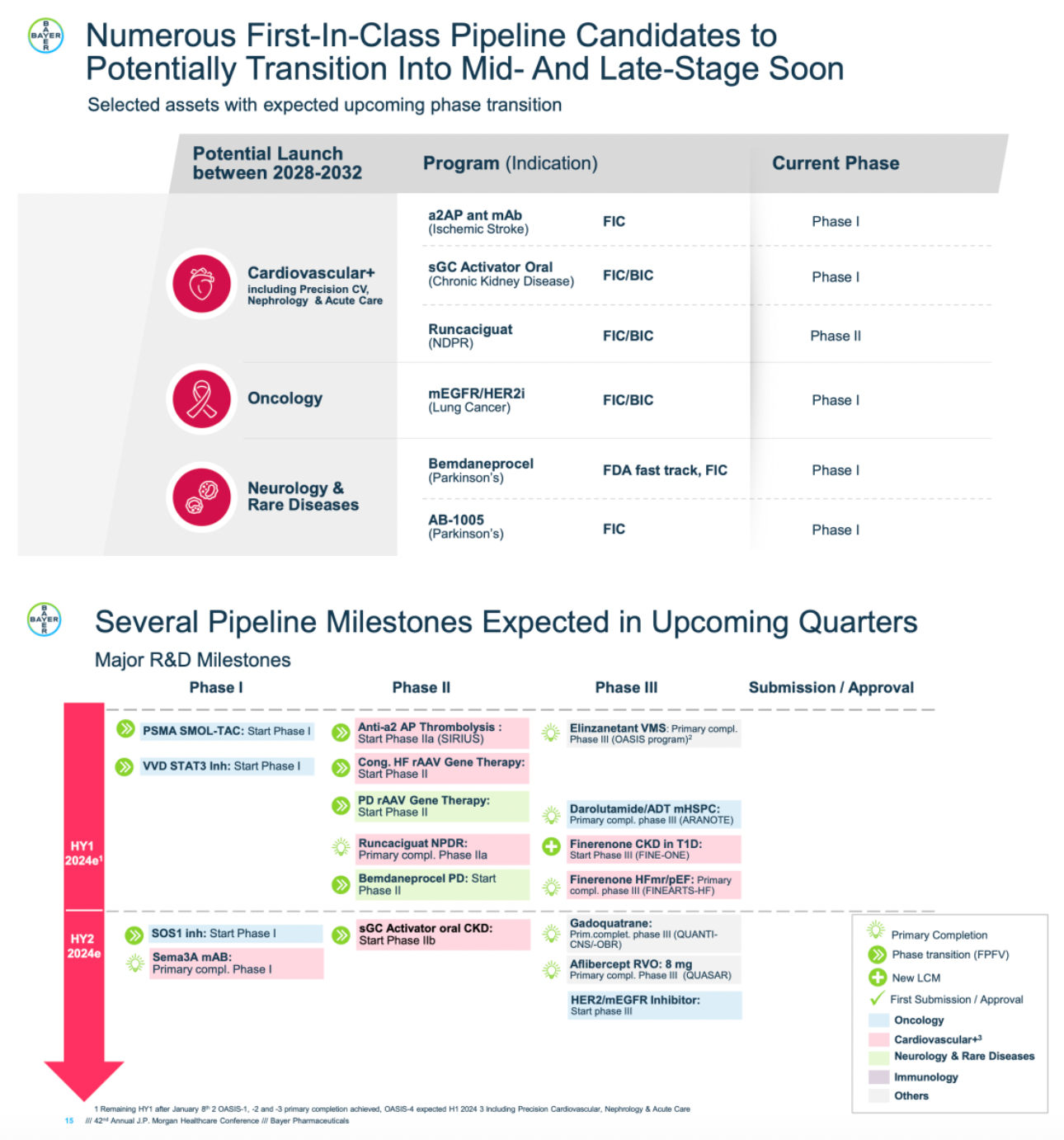
Elinzanetant is being explored as a non-hormonal therapeutic option for menopause with significant potential. It is a dual NK1 and NK3 receptor antagonist currently in Phase III clinical trials. The OASIS clinical trial program for Elinzanetant has two pivotal Phase III studies that have met all primary and key secondary endpoints.
BAY-3375968 is a monoclonal antibody targeting the CCR8 receptor and is being clinically developed for the treatment of solid tumors. In vitro studies have shown that BAY-3375968 can selectively deplete human CCR8-positive Tregs through antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).
BAY-3178275 is an antagonist targeting GPR84 and is undergoing Phase I clinical development as a treatment for diabetic neuropathic pain.
Gilead in JPM 2024
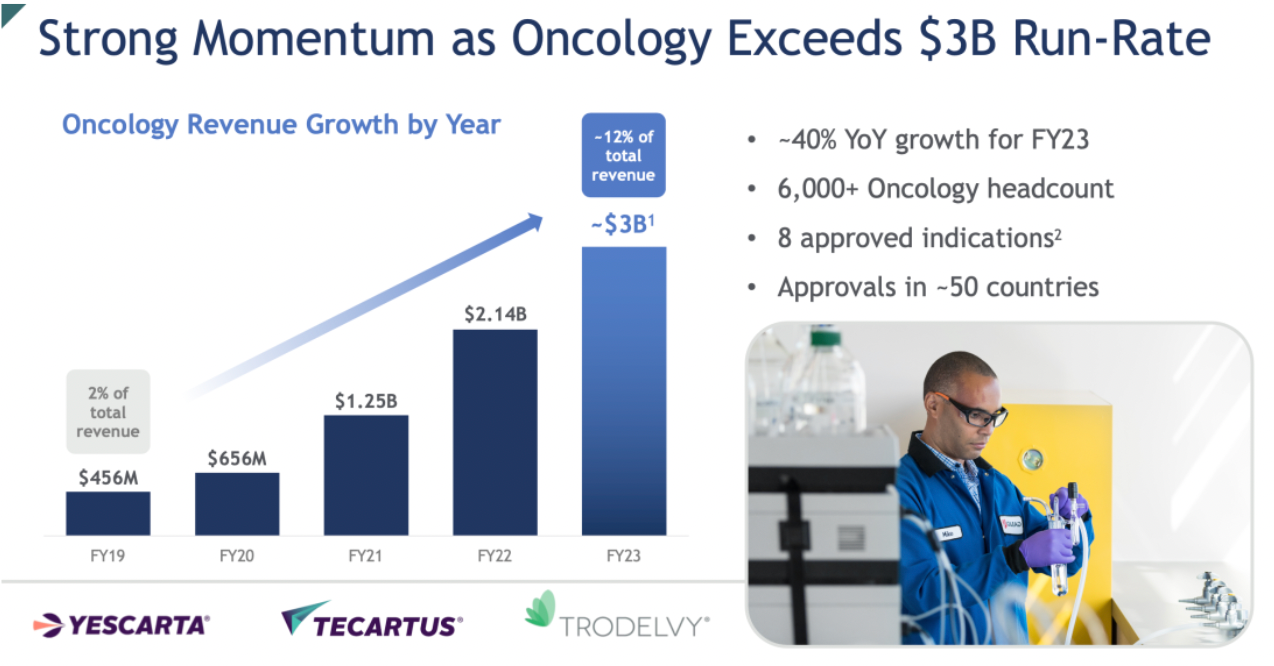
Gilead's robust execution in 2023 has become a catalyst for 2024, accelerating the development of its oncology business while maintaining sustained growth in its leading portfolio of HIV products.
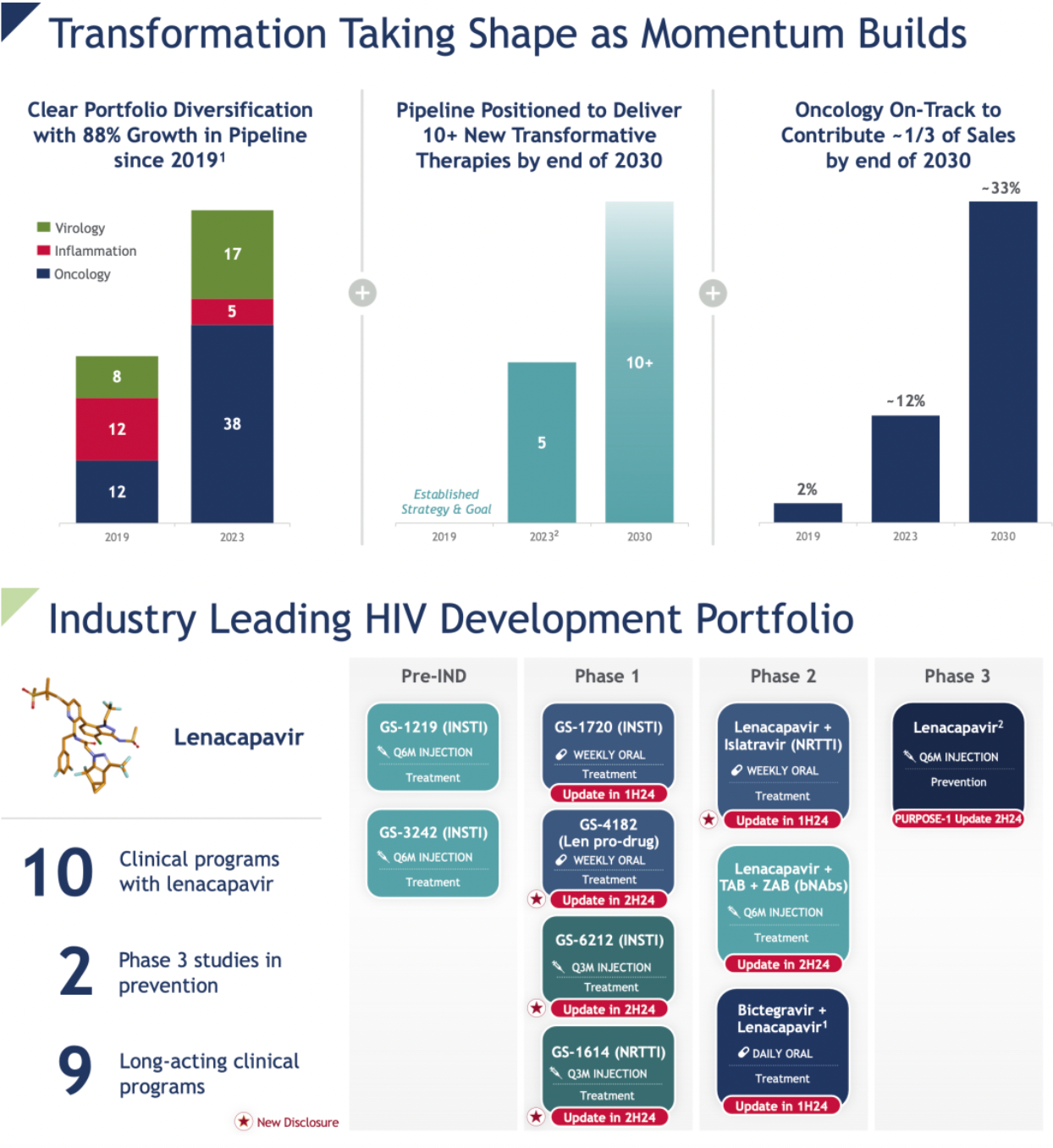
Gradually establishing leadership in cell therapy and CAR-T direction.

In 2024, we will see updates across multiple research and development pipelines.

GS-1811 is an inhibitor targeting CCR8 and is currently being developed clinically for the treatment of solid tumors. GS-1811 is intended to selectively deplete tumor-infiltrating regulatory T cells, which exert immunosuppressive effects within the tumor microenvironment. It is presently in Phase 1 clinical development. This drug may potentially be used in combination with multiple Gilead medications, such as Trodelvy.
GS-6201 is a selective small molecule antagonist targeting the A2bR receptor. It is undergoing Phase 1 clinical trials for conditions including lung cancer.