GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (5)
In this article, we will focus on Sosei Heptares, a leading company in the field of GPCR new drug development, and its GPCR research and development pipeline. For more details, please follow the link below.
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (1)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (2)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (3)
- GPCR Targets and Pipeline at the JP Morgan Healthcare Conference 2024 (4)
Sosei Heptares in JPM 2024
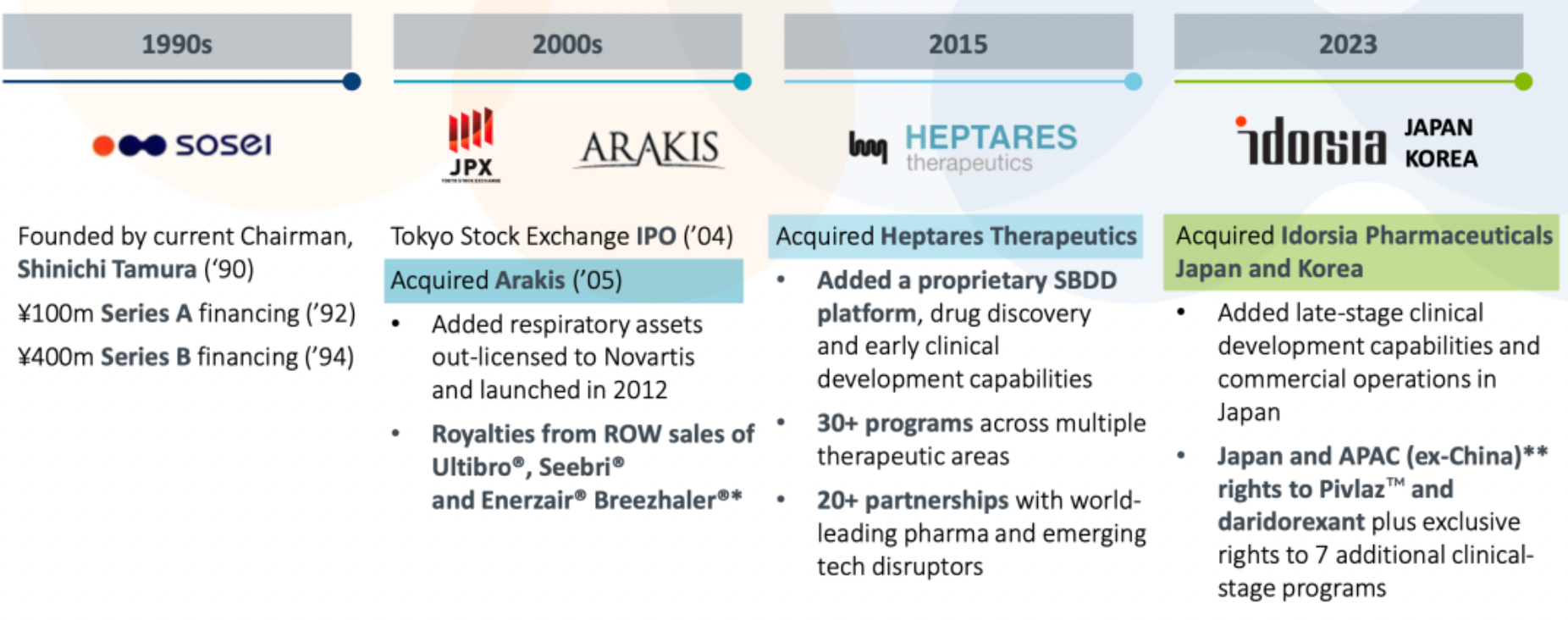
Sosei Heptares is a biopharmaceutical company based in Japan with operations spanning the Asia-Pacific region, focused on structure-based drug design (SBDD), particularly in the area of G protein-coupled receptors (GPCRs). The company has over 30 active research and development projects involving central nervous system disorders, immunology, gastrointestinal diseases, and various other therapeutic areas. Its marketed product, PIVLAZ®, is widely used in Japan and South Korea, where it has been approved for use.

Over the past 20 years, Sosei has undergone several rounds of acquisitions and restructurings and completed a significant transformation in 2023, including the acquisition of Idorsia Pharmaceuticals' operations in Japan and the Asia-Pacific region and securing significant investments from government funds. Additionally, the company has collaborated with several top global pharmaceutical companies, making clinical advancements in the treatment of metabolic and neuropsychiatric disorders such as insomnia. Daridorexant is expected to be launched in Japan by the end of 2024.
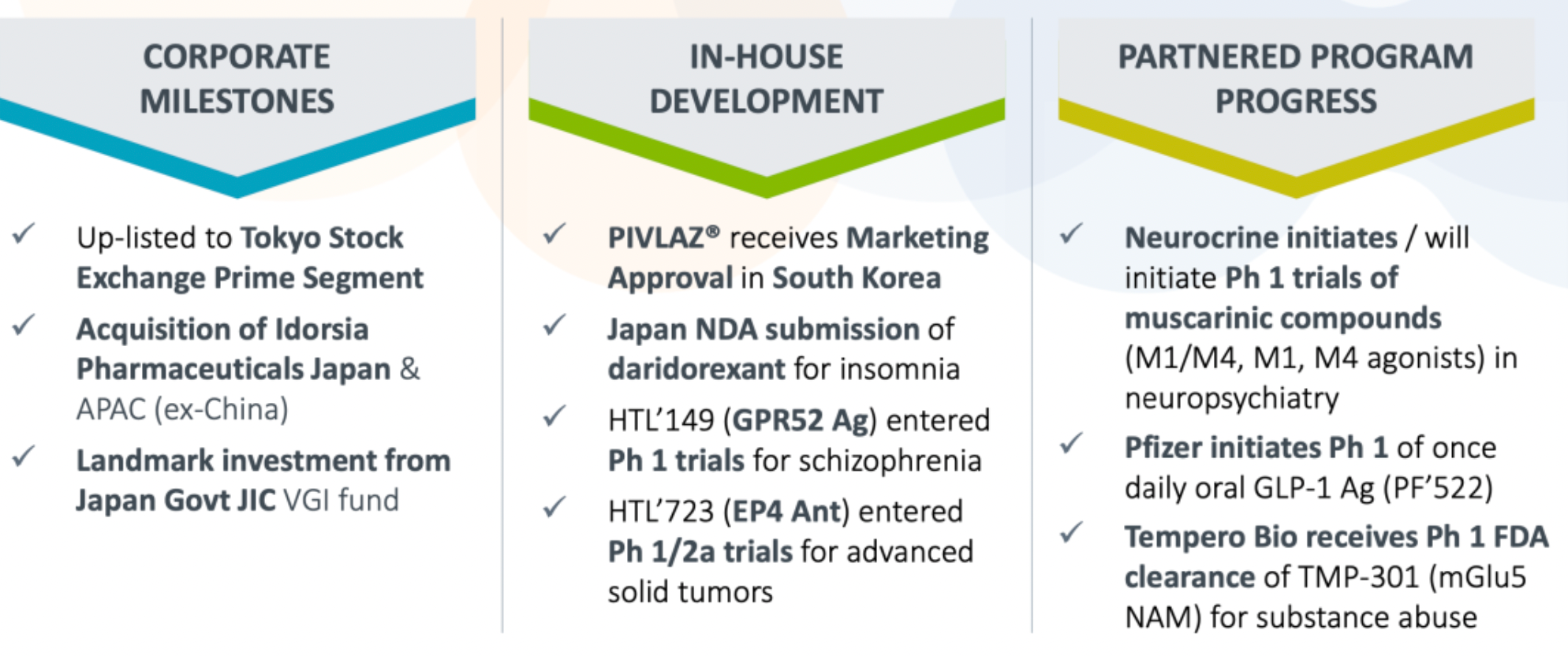
In 2023, Sosei Heptares has implemented a series of corporate actions and investments to drive future growth. The company's internal research projects, including PIVLAZ®, daridorexant, HTL-149 (a GPR52 agonist), and HTL-723, have achieved milestone progress. Moreover, Sosei Heptares and its partners launched multiple clinical programs in 2023, involving the research and development of several innovative therapies for metabolic and neuropsychiatric disorders. This includes a range of novel cholinergic M4 and M1/M4 agonists being developed in collaboration with Neurocrine for the study of schizophrenia and other neuropsychiatric diseases, with some drugs nearing or having entered the late clinical phase.
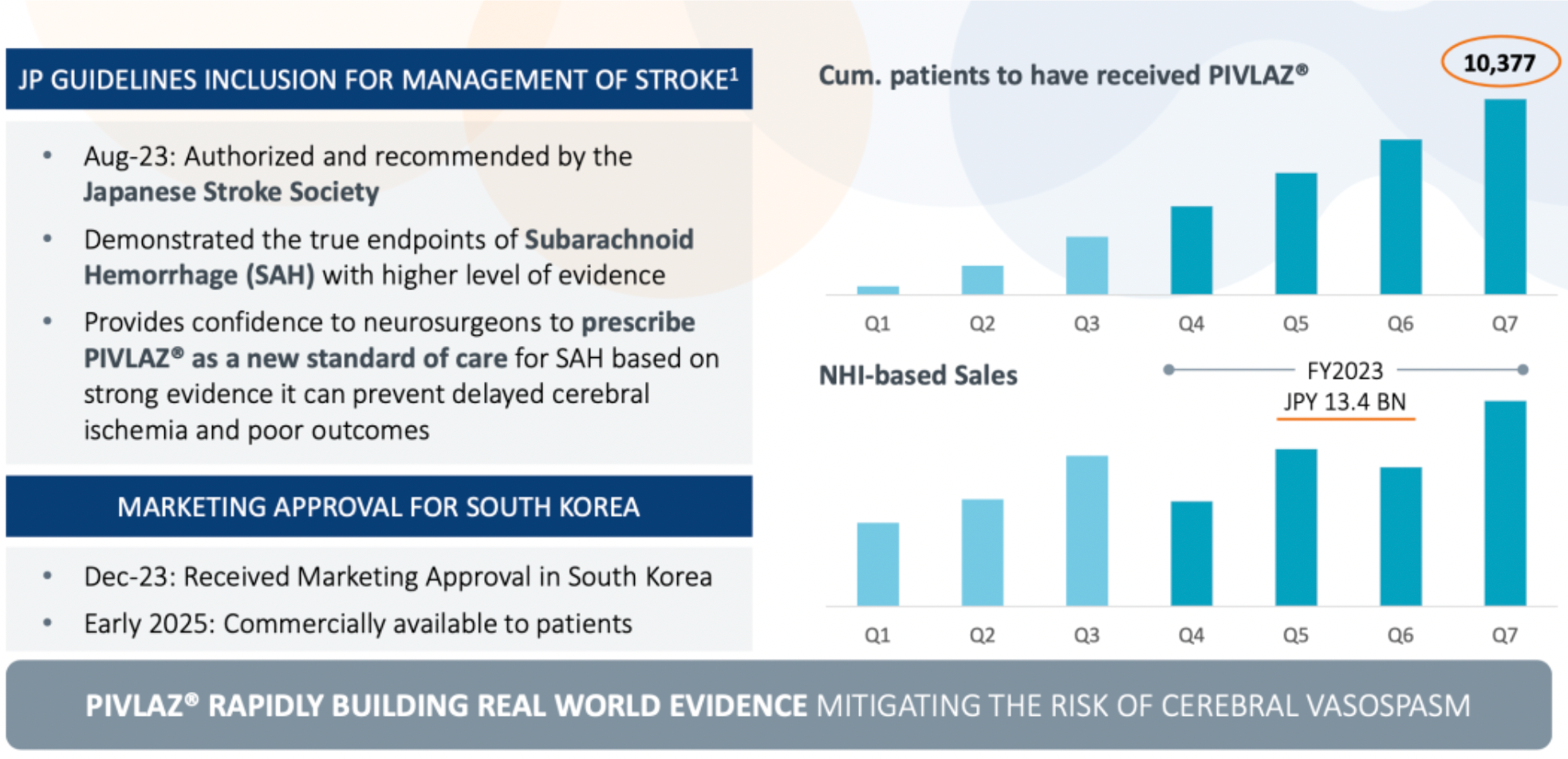
The company's first commercially available medicine, PIVLAZ® (a selective ETA receptor antagonist), has made a significant impact on stroke treatment in Japan and has been accumulating evidence in the real world. PIVLAZ® is recommended by the Japan Stroke Society and has proven to have a definitive therapeutic effect on subarachnoid hemorrhage (SAH), preventing delayed cerebral ischemia and adverse outcomes with a high level of evidence, thus strengthening the confidence of neurosurgeons in prescribing the medication. Additionally, the drug has received market approval in Korea and is planned to start sales to patients at the beginning of 2025.
By the end of 2023, the company has submitted a New Drug Application (NDA) in Japan for daridorexant for the treatment of insomnia, with an anticipated launch in the Japanese market in the fourth quarter of 2024. The drug is expected to make a significant contribution to Japanese society. Insomnia causes an estimated annual economic loss of up to 15 trillion yen in Japan (approximately 3% of GDP), and results in the loss of 604,000 working days due to absenteeism and medical leave. Moreover, individuals averaging less than 6 hours of sleep per night face an increased mortality risk by 10%. Daridorexant, a dual orexin receptor antagonist, is already on the market in the United States and Europe under the brand name QUVIVIQ™. Joint development and co-marketing plans for daridorexant in Japan are currently underway, with expectations to effectively address the issue of insomnia.

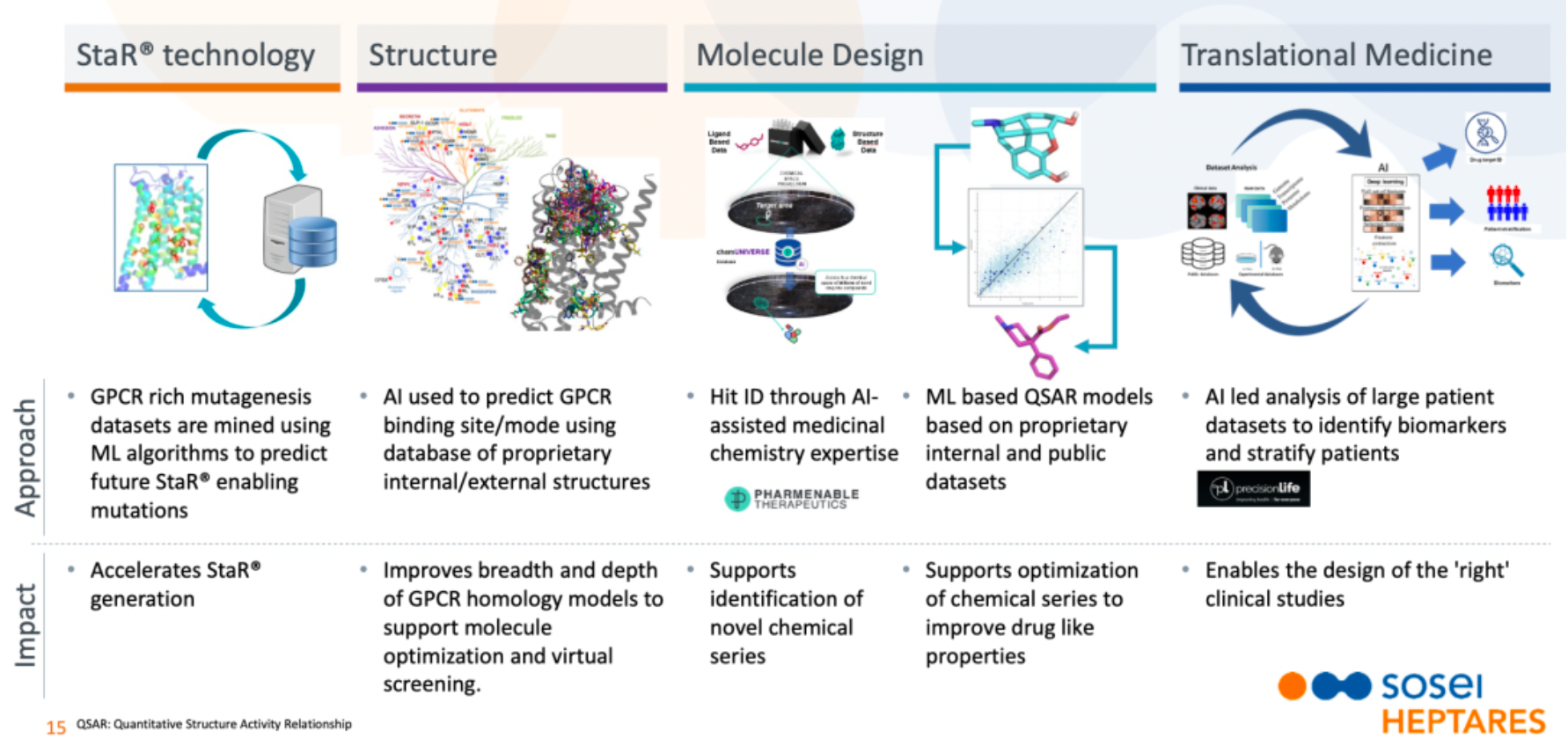
Sosei Heptares employs Artificial Intelligence (AI) and Machine Learning (ML) technologies in various drug development processes to enhance the efficiency of drug discovery, including receptor preparation technology (StaR®), protein structure, molecular design, and translational medicine. Utilizing the StaR® technology in combination with AI/ML algorithms, the company mines datasets rich in GPCR mutations to predict and accelerate the generation of StaR® variants conducive to stabilizing GPCR structures, thereby hastening the drug discovery process. Additionally, AI technology is used to analyze GPCR binding sites and patterns, enhancing the accuracy of GPCR homology models to support drug molecule optimization and virtual screening efforts. Hit identification is conducted based on internal and external structural databases, while drug molecule designs are optimized using ML QSAR models derived from internal and public datasets. Moreover, AI is applied to analyze large patient datasets to identify biomarkers and stratify patients, ensuring more precise and effective clinical trial designs. The application of these advanced technologies not only aids in the identification of new chemical series but also enhances the optimization of existing chemical series, improving drug properties with the goal of developing more life-changing medicines.
The company possesses several internally researched and collaboratively developed GPCR (G protein-coupled receptor) new drug development pipelines, which are at various stages of research and development. These pipelines encompass multiple therapeutic areas including metabolic diseases, inflammatory bowel disease, schizophrenia and neuropsychiatric disorders, as well as solid tumors.
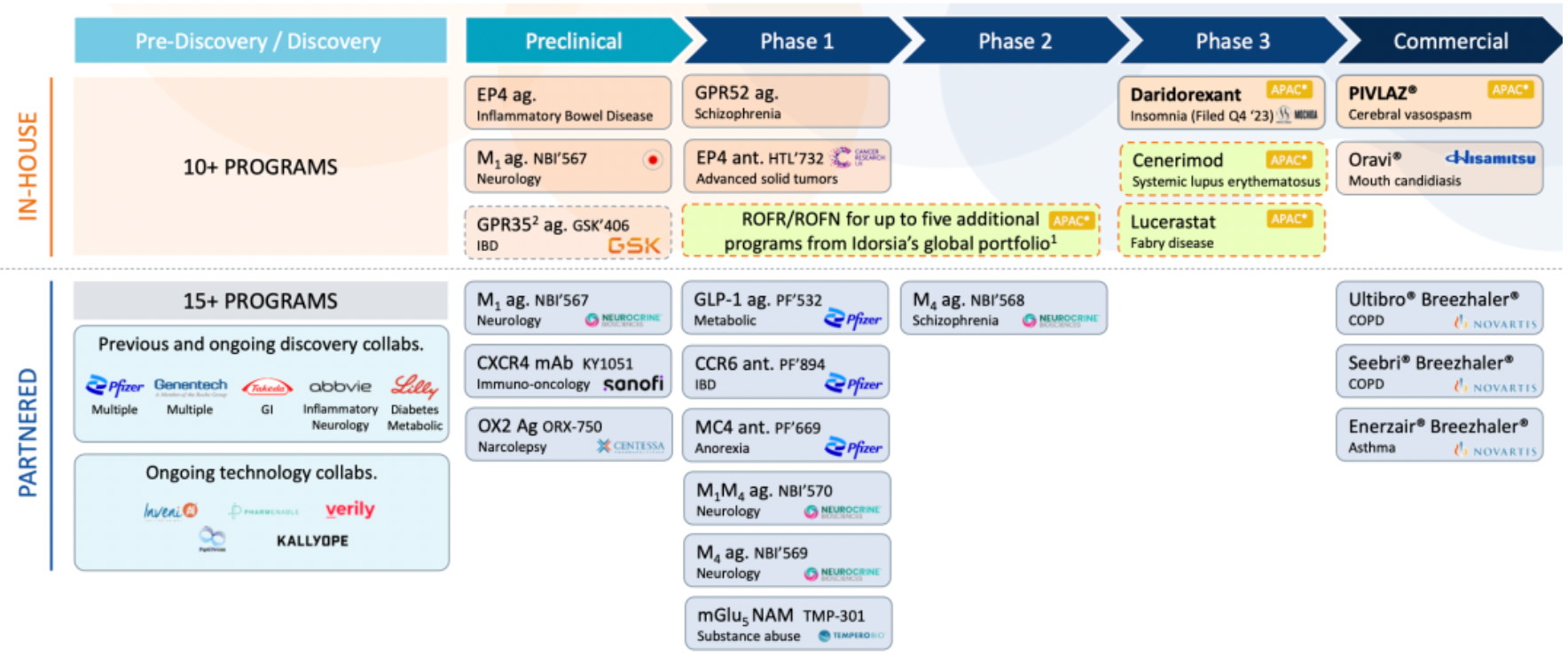
PIVLAZ® (Clazosentan) is a potent, selective endothelin ETA receptor antagonist indicated for the prevention of cerebral vasospasm, vasospasm-related cerebral infarction, and ischemic symptoms following aneurysmal subarachnoid hemorrhage (aSAH). Pivlaz® successfully launched in Japan in April 2022 for the treatment of cerebral vasospasm. Sosei Heptares holds the rights to sell Pivlaz® in Japan and the Asia-Pacific region, excluding China.
Ultibro Breezhaler is a pioneering inhaled dual bronchodilator (long-acting β2-adrenergic receptor agonist or long-acting muscarinic antagonist, LAMA/LABA), approved in more than 90 countries including the European Union and Japan. It is a once-daily fixed-dose combination formulation of indacaterol and glycopyrronium bromide, indicated in the European Union for the maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD).
Daridorexant is a dual orexin receptor antagonist that blocks the binding and activity of the wakefulness-promoting neuropeptide orexin. In October 2022, daridorexant achieved positive Phase 3 clinical trial results among patients with insomnia in Japan. The success of the trial paved the way for regulatory submission in Japan in the second half of 2023. Daridorexant is approved in the United States and Europe and is marketed by Idorsia under the brand name QUVIVIQ™ in these regions. Sosei Heptares has the rights to sell daridorexant in Japan and the Asia-Pacific region (excluding China).
Cenerimod is an S1PR1 modulator currently under Phase 3 clinical investigation for the treatment of systemic lupus erythematosus (SLE). Cenerimod aims to reduce autoimmune responses and control disease activity in patients with SLE by affecting lymphocyte migration. In its collaboration with Idorsia, Sosei Heptares has obtained not only the two key products, PIVLAZ® and daridorexant, but also exclusive options to license cenerimod and lucerastat, as well as selection rights for five additional projects in Idorsia's global pipeline.
HTL0016878 is a selective oral small-molecule muscarinic M4 receptor agonist currently in Phase II clinical development for the treatment of schizophrenia. The mechanism of action of HTL0016878 differs from existing antipsychotic medications, and its high selectivity compared to previous non-selective muscarinic receptor agonists offers the potential for improved safety.
The GPR52 agonist program is currently in Phase I clinical research, targeting schizophrenia as an indication. GPR52 is an orphan GPCR expressed in the central nervous system, implicated in neural development and regulation of the dopaminergic system. As a potential therapeutic approach, GPR52 agonists aim to ameliorate schizophrenia-related psychopathological symptoms by targeting this receptor.
HTL0039732 is a novel, highly selective, and potent EP4 receptor antagonist intended for the clinical treatment of advanced solid tumors by mediating the immunosuppressive effects of PGE2 within the tumor microenvironment. In July 2022, Sosei Heptares signed an agreement with Cancer Research UK to bring Sosei Heptares' cancer immunotherapy candidate into its first human trials.
Lotiglipron (PF-07081532) is an oral small-molecule GLP-1R agonist co-developed by Sosei Heptares and Pfizer. Initiated by its partner Pfizer, the Phase I clinical trial of this once-daily oral GLP-1 receptor agonist is expected to improve the metabolic status of patients with diabetes and obesity. At the end of 2022, Sosei Heptares and Pfizer announced the advancement of Lotiglipron (PF-07081532) to Phase II clinical trials. However, in June 2023, Pfizer announced the discontinuation of the clinical development of PF-07081532. At the end of November 2023, Pfizer announced the inclusion of another GLP-1R agonist, PF-06954522, developed in collaboration with Sosei Heptares, into Phase I clinical research.
PF-07054894 is a selective small molecule antagonist targeting CCR6 for clinical treatment of inflammatory bowel disease (IBD). This project is also part of a collaborative research program developed with Pfizer.
PF-07258669 is a selective antagonist targeting the MC4R receptor co-developed by Sosei Heptares and Pfizer, and is being clinically developed for the treatment of cachexia associated with cancer. NBI-1117570 is a dual agonist product selectively targeting the M1/M4 receptors, intended for the treatment of a variety of psychiatric disorders in the neuropsychiatric field, including but not limited to diseases related to cognition, mood, and behavior. This drug is currently in a Phase 1 clinical development study in collaboration with Neurocrine.
NBI-1117569 is a selective small molecule agonist targeting the M4 receptor, while NBI-1117567 is a selective small molecule agonist targeting the M1 receptor. Both different cholinergic receptor agonists were developed using Sosei Heptares' structure-based drug design platform and are involved in neuropsychiatric indications at different stages of research. The former is in a Phase 1 clinical trial, and the latter is ready to enter Phase 1 clinical study; both drugs are being co-developed with Neurocrine.
HTL0014242 (TMP-301) is a potent, selective negative allosteric modulator of the mGlu5 receptor discovered by Sosei Heptares, used for the treatment of certain neurological disorders. It is currently in a Phase 1 collaborative development by Tempero Bio for the treatment of substance abuse issues.
The EP4 agonist project is currently in the preclinical research phase with indications for inflammatory bowel disease (IBD). The drug is an oral, once-daily small molecule formulation designed to modulate the prostaglandin E2 EP4 receptor subtype within the gastrointestinal tract. By activating the EP4 receptor, the medication may help to regulate immune responses and inflammation processes, thereby alleviating the symptoms of IBD patients.
GSK4381406 is a GPR35 receptor agonist co-developed by Sosei Heptares and GSK for the clinical treatment of inflammatory bowel disease, currently in phase 1 clinical research. In November last year, Sosei Heptares announced that, due to GSK's strategic realignment, the company would independently continue the research on the GPR35 receptor agonist project returned by GSK. This orally administered, gut-restricted new drug has the potential to improve intestinal barrier function and reduce visceral pain sensitivity, with plans to re-initiate entry into phase 1 clinical trials.