InflaRx Begins Phase 2a Trial of Oral C5aR Inhibitor INF904 with First Patient Treatment
InflaRx N.V. (Nasdaq: IFRX), a biopharmaceutical firm focusing on developing anti-inflammatory treatments that target the complement system, has reported the administration of the first dose to a patient participating in its Phase 2a basket trial for chronic spontaneous urticaria (CSU) and hidradenitis suppurativa (HS). This study is evaluating the Company’s oral C5aR inhibitor, INF904.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Dr. Camilla Chong, the Chief Medical Officer at InflaRx, stated: “We are excited to announce the initiation of our Phase 2a trial for INF904, with the first patient having received a dose at one of our U.S. locations. I am extremely proud of our team for executing this crucial study in such a timely manner. We are confident that there is substantial evidence supporting the anti-inflammatory effects of INF904 and its potential to address significant medical needs in chronic spontaneous urticaria (CSU), hidradenitis suppurativa (HS), and other immuno-inflammatory disorders.”
The Phase 2a trial is a multi-center, open-label investigation aiming to enroll a total of 75 patients suffering from moderate-to-severe forms of CSU and HS. The study will assess different dosing strategies of INF904 over a four-week treatment duration to collect further safety and pharmacokinetic (PK) information, alongside indicators of clinical efficacy. As previously discussed, this study will use a commercially viable formulation of INF904, allowing for a range of drug exposure similar to that reported in the Phase 1 trial. Following the four-week treatment, patients will continue to be monitored for another four weeks. Results from this trial are anticipated in the summer of 2025, with an intention to guide the framework of a larger, longer-term Phase 2b study by the end of 2025.
Within the CSU cohort, 45 patients will receive treatment across three arms of the study. Participants in Arms 1 and 2 will be randomly assigned at a 1:1 ratio to receive either 60 mg or 120 mg of INF904 twice daily (BID), mirroring the dosage exposures noted in the Phase 1 study. Those in Study Arm 3 will consist of patients who did not respond to anti-IgE treatments, and will receive a dose of 120 mg BID. Alongside safety and PK evaluations, efficacy metrics for CSU will involve changes in the Urticaria Activity Score 7 (UAS7), Hives Severity Score (HSS7), and Itch Severity Score (ISS7) from the baseline until the conclusion of week 4. Additionally, responder analysis, biomarker assessments, and Patient-Reported Outcome (PRO) measures concerning urticaria management and overall quality of life will also be evaluated.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
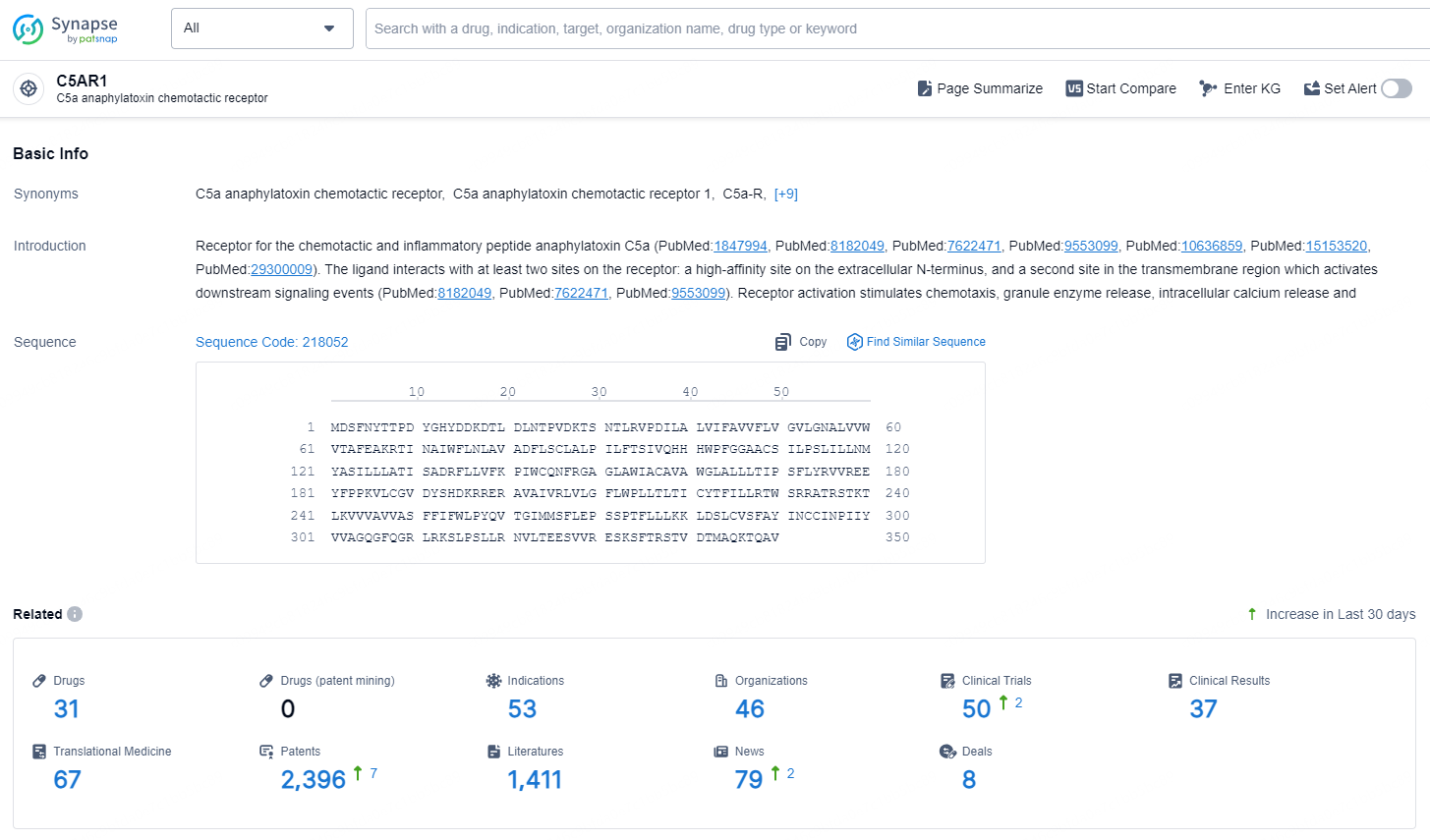
According to the data provided by the Synapse Database, As of December 26, 2024, there are 31 investigational drugs for the C5AR1 target, including 53 indications, 46 R&D institutions involved, with related clinical trials reaching 50, and as many as 2396 patents.
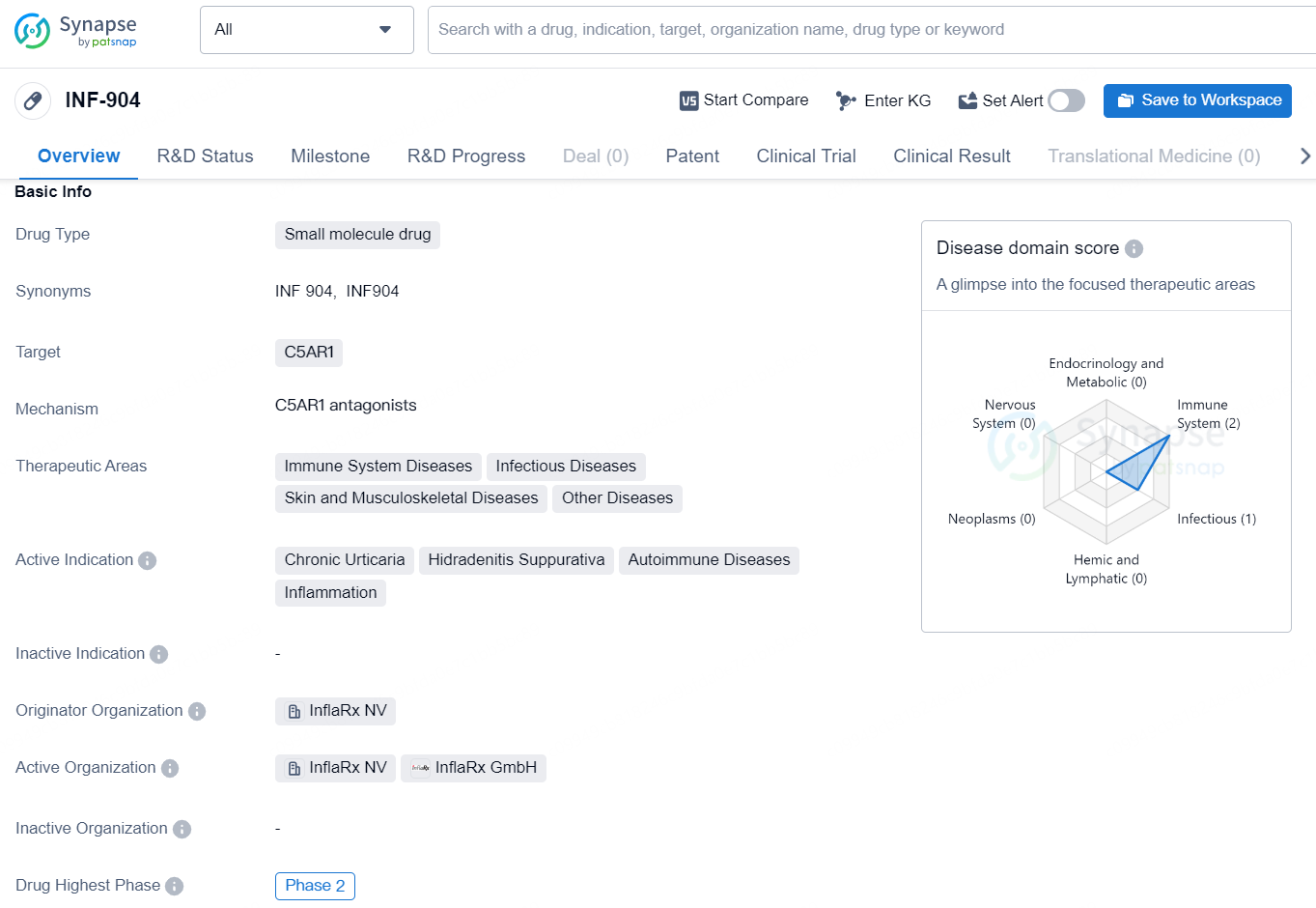
INF-904 is a small molecule drug developed by InflaRx NV, designed to target the C5AR1 receptor in the treatment of various conditions. The drug falls within the therapeutic areas of immune system diseases, infectious diseases, and skin and musculoskeletal diseases, in addition to being explored as a potential treatment for other diseases. The active indications for INF-904 include chronic urticaria, hidradenitis suppurativa, autoimmune diseases, and inflammation.