Oruka Therapeutics Begins Phase 1 Trial Dosing with New IL-23p19 Antibody, ORKA-001
Oruka Therapeutics, Inc. (“Oruka”) (Nasdaq: ORKA), a biotech firm focused on creating innovative biologics aimed at establishing a new benchmark for managing chronic skin disorders like plaque psoriasis, has revealed the commencement of dosing healthy volunteers in its inaugural clinical trial for ORKA-001. This novel monoclonal antibody, administered subcutaneously and designed with an extended half-life, specifically targets IL-23p19.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"The launch of the Phase 1 trial for ORKA-001 is a significant milestone for Oruka, achieved ahead of our planned schedule," stated Lawrence Klein, PhD, Chief Executive Officer of Oruka. "We are eager to present preliminary data for ORKA-001 in the latter part of 2025, which may affirm the drug's half-life and safety profile, potentially enabling longer dosing intervals and establishing it as a leading treatment option."
The Phase 1 ORKA-001 study is designed as a double-blind, placebo-controlled trial involving single ascending doses to assess the safety, tolerability, and pharmacokinetics (PK) of ORKA-001 in a group of healthy participants. Approximately 24 healthy volunteers will be enrolled across three subcutaneous dosing groups. Oruka anticipates releasing interim findings from this trial in the latter half of 2025.
Based on the results of the Phase 1 study, Oruka intends to commence a proof-of-concept trial for ORKA-001 targeting moderate-to-severe psoriasis in the latter half of 2025. This trial is expected to assess both safety and efficacy of a single dosage of ORKA-001 compared to a placebo in around 80 participants, followed by their random assignment to one of two maintenance dosing strategies. One strategy will involve administering ORKA-001 every six months, while the other will consist solely of induction doses to evaluate how long patients can maintain clear skin, which could accommodate annual dosing or even longer durability for some individuals. Participants may also transition into an open-label extension study. The company anticipates sharing initial outcomes from the proof-of-concept trial in the second half of 2026.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
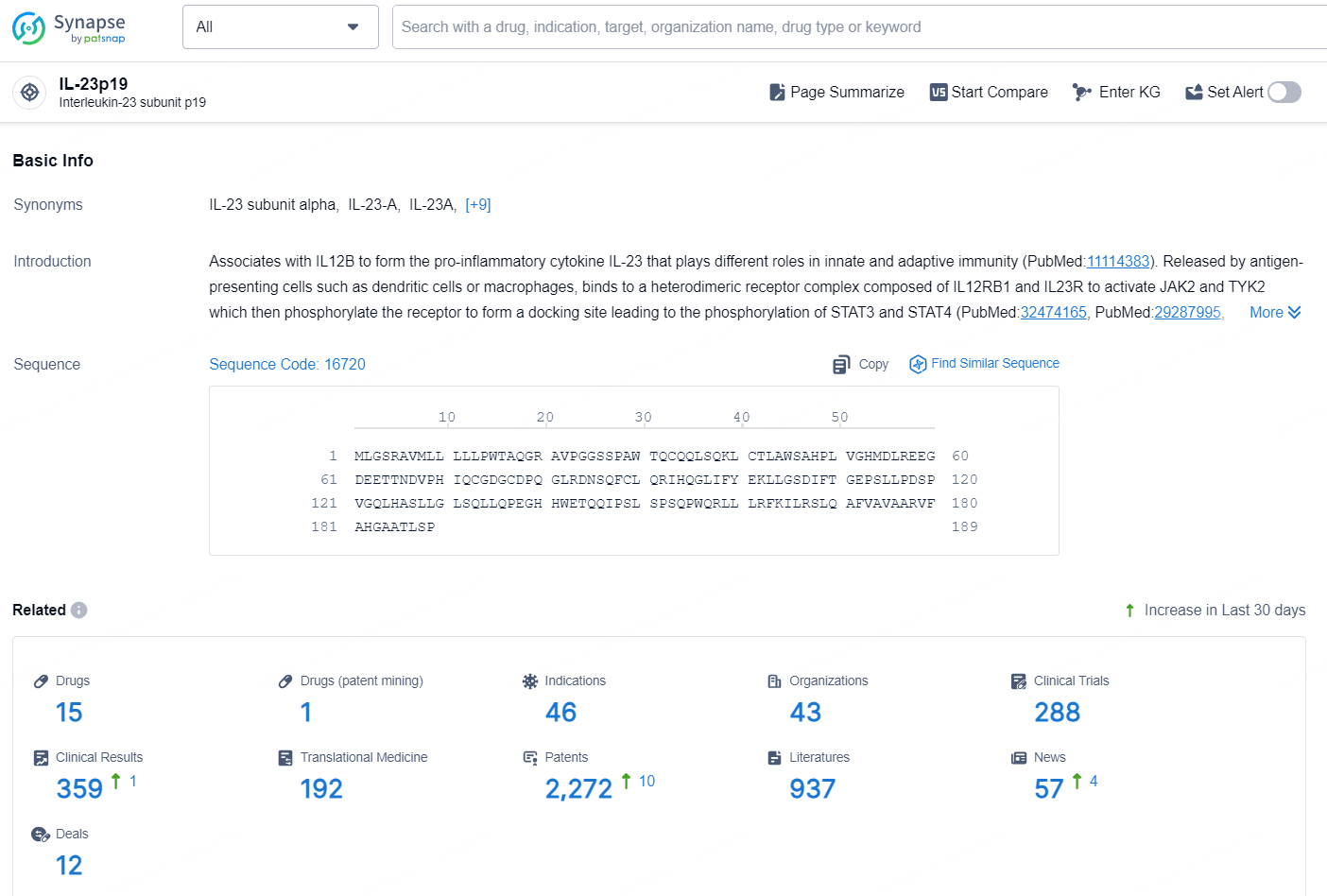
According to the data provided by the Synapse Chemical, As of December 24, 2024, there are 15 investigational drugs for the IL-23p19 target, including 46 indications, 43 R&D institutions involved, with related clinical trials reaching 288, and as many as 2272 patents.
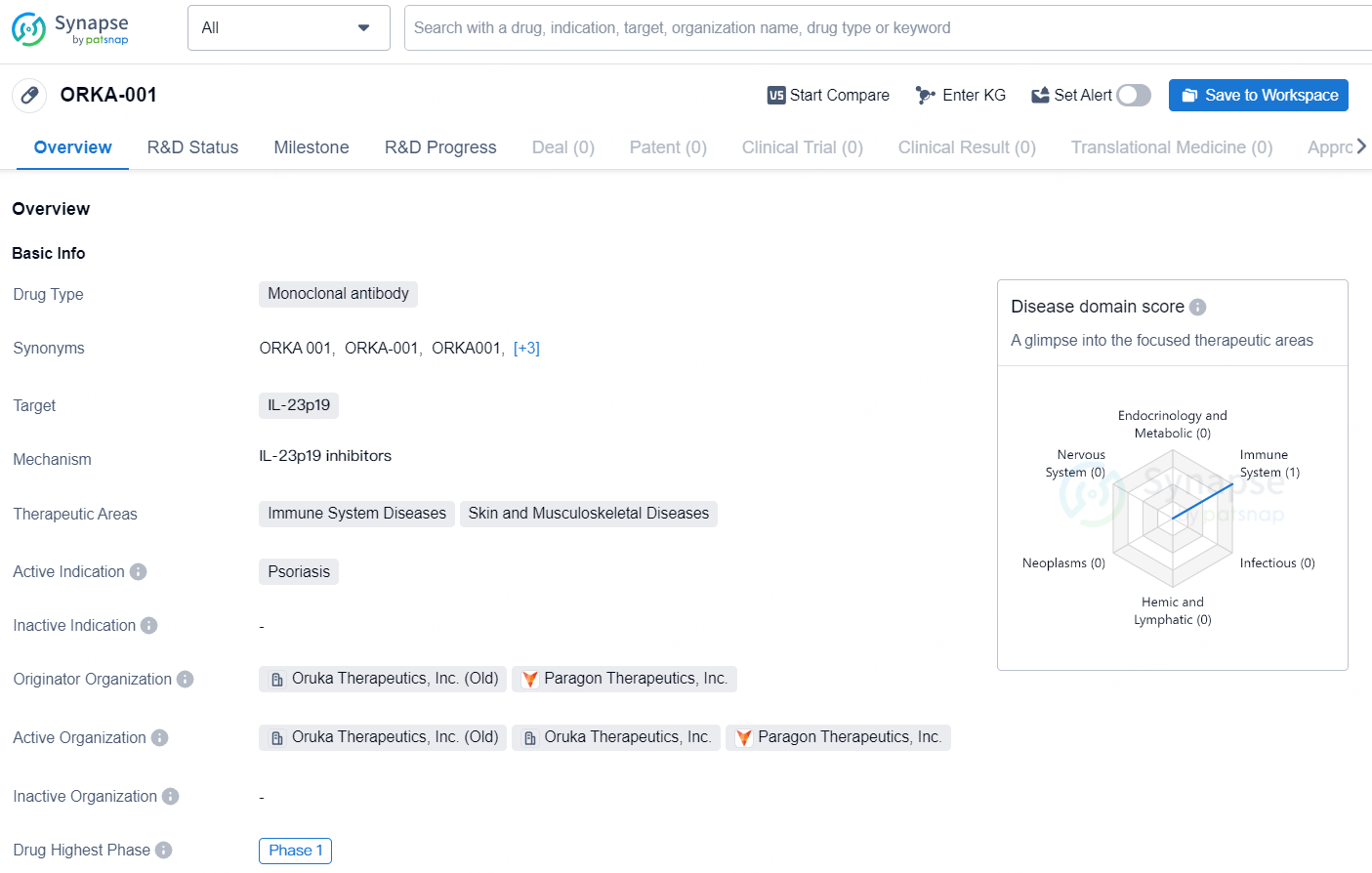
ORKA-001 is a monoclonal antibody drug that targets IL-23p19 and is primarily focused on treating immune system diseases, skin, and musculoskeletal diseases. The active indication for the drug is psoriasis. Oruka Therapeutics, Inc. (Old) and Paragon Therapeutics, Inc. are the originator organizations of this drug. Currently, ORKA-001 is in Phase 1, which is the highest phase of its global development.