Regeneron Advances Two Factor XI Antibodies to Phase 3 Following Successful Phase 2 Results
Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN) has announced encouraging results from Phase 2 trials involving two innovative monoclonal antibodies that focus on different regions of Factor XI. REGN7508, which targets the catalytic domain, aims to enhance anticoagulant efficacy while reducing the risk of bleeding. In contrast, REGN9933, which interacts with the A2 domain, is intended to offer an alternative for patients facing the highest bleeding risks, who may not be suitable for existing anticoagulant therapies. According to the findings from the Phase 2 trials, both antibodies demonstrated a significant antithrombotic effect, and no clinically significant bleeding incidents were noted across the treatment groups.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“Our Factor XI antibodies, which focus on the catalytic and A2 domains, were thoroughly assessed against existing treatment standards and demonstrated a significant antithrombotic effect along with an encouraging safety profile following a single convenient dose,” stated George D. Yancopoulos, M.D., Ph.D., Board Co-Chair, President, and Chief Scientific Officer at Regeneron. “The recent Phase 2 findings complement our preclinical data, which indicated that the prolongation of activated partial thromboplastin clotting time was more significant with REGN7508 and comparable with REGN9933, in relation to other Factor XI inhibitors. The combination of these clinical and preclinical results, along with compelling genetic insights, bolsters our confidence in the potential of targeting multiple unique domains of Factor XI, enabling us to potentially provide customized therapies for patients with varying bleeding risks across diverse treatment scenarios. We are excited to progress REGN7508 and REGN9933 into an extensive Phase 3 trial starting in 2025.”
Regeneron executed two open-label, active-controlled Phase 2 trials (ROXI-VTE-I and ROXI-VTE-II) at the same institutions using similar methodologies to assess REGN7508 and REGN9933 for the prevention of asymptomatic (identified via venogram between days 8 and 12) or symptomatic venous thromboembolism (VTE) following unilateral total knee arthroplasty. In ROXI-VTE-I, participants were randomly assigned to receive either a single intravenous (IV) dose of REGN9933, daily enoxaparin, or apixaban doses twice daily until venography. In ROXI-VTE-II, subjects were randomly assigned to receive a single IV dose of REGN7508 or daily enoxaparin until the venography. Unlike trials assessing other Factor XI antibodies, all treatments in these trials commenced 12 to 24 hours post-surgery (typically one day after the operation), aligning with the approved timing for administering the active comparators.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
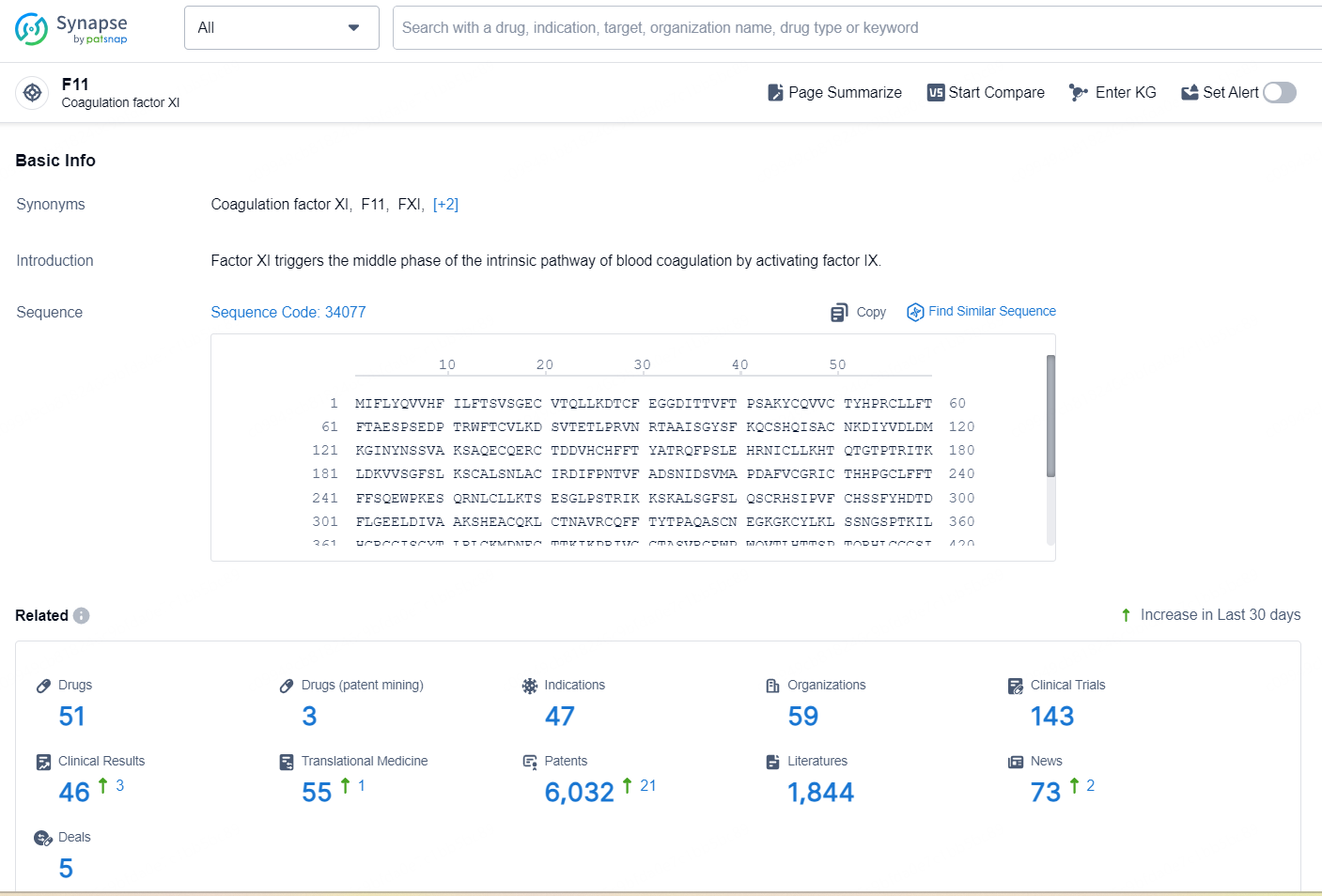
According to the data provided by the Synapse Database, As of December 24, 2024, there are 51 investigational drugs for the F11 target, including 47 indications, 59 R&D institutions involved, with related clinical trials reaching 143, and as many as 6032 patents.
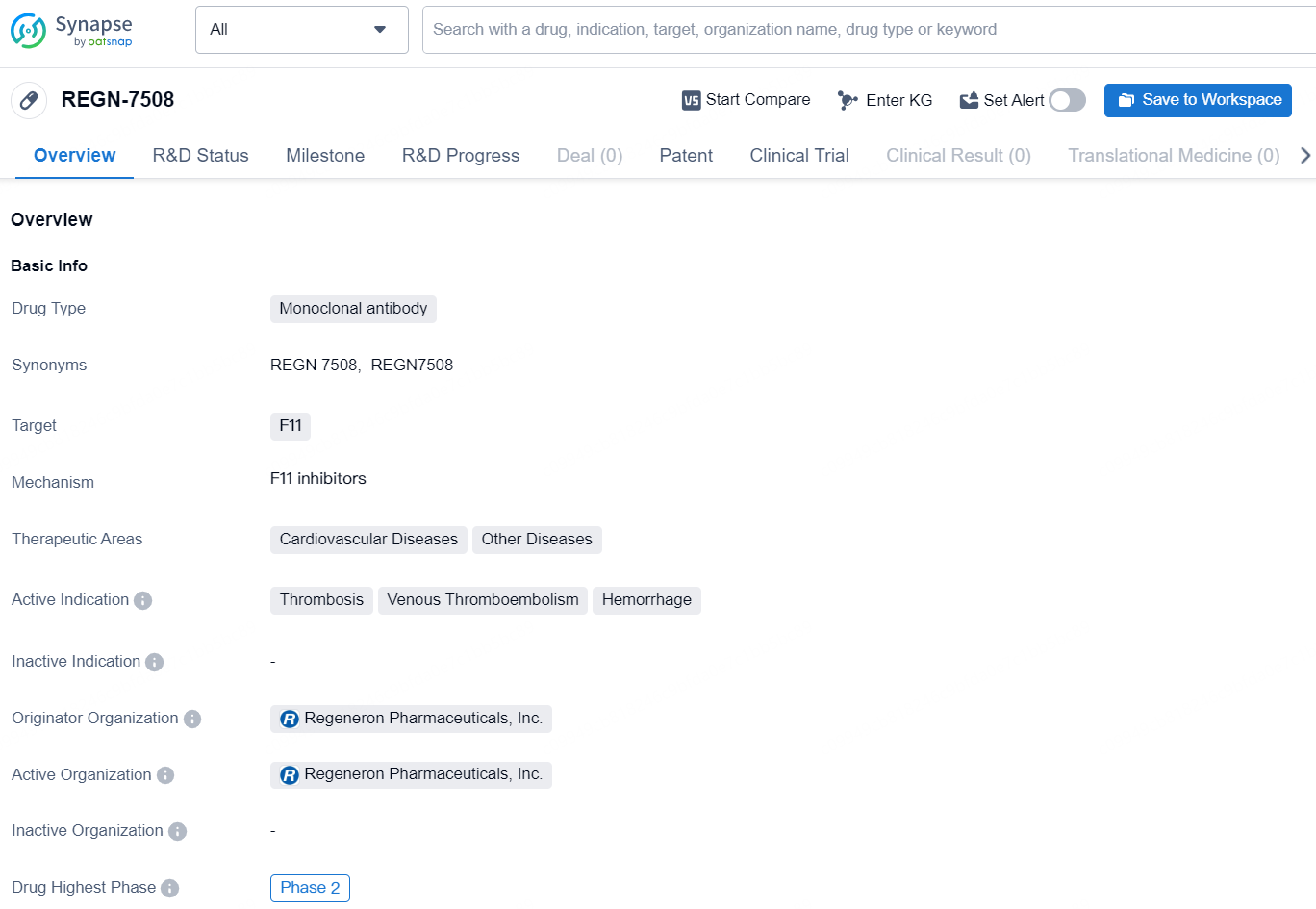
REGN-7508 is a monoclonal antibody drug developed by Regeneron Pharmaceuticals, Inc. The drug targets F11 and is being developed for the treatment of various cardiovascular diseases and other related conditions. Its active indications include thrombosis, venous thromboembolism, and hemorrhage.