Novo Nordisk's CagriSema Leads to Superior Weight Loss in REDEFINE 1 Trial
Novo Nordisk has released key findings from REDEFINE 1, a phase 3 study that is part of the global REDEFINE program. This study, lasting 68 weeks, assesses the efficacy and safety of subcutaneous CagriSema (a fixed-dose combination of cagrilintide 2.4 mg and semaglutide 2.4 mg) in comparison to its individual components: cagrilintide 2.4 mg, semaglutide 2.4 mg, and a placebo, all administered weekly. The trial enrolled 3,417 individuals who are either obese or overweight and have one or more comorbid conditions, with an average baseline body weight of 106.9 kg.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The study met its primary objective by showing a statistically significant and enhanced weight reduction at week 68 with CagriSema in comparison to placebo.
The REDEFINE 1 trial utilized a flexible protocol, permitting participants to adjust their dosages during the study. After 68 weeks, 57.3% of those receiving CagriSema were on the highest dose, whereas 82.5% were using cagrilintide 2.4 mg and 70.2% were on semaglutide 2.4 mg.
When considering treatment effects under the assumption that all individuals adhered to the therapy1, participants administered CagriSema experienced a weight loss of 22.7% after 68 weeks, significantly exceeding the 11.8% loss with cagrilintide 2.4 mg, 16.1% with semaglutide 2.4 mg, and 2.3% with placebo. Additionally, 40.4% of individuals treated with CagriSema achieved a weight loss of 25% or greater after 68 weeks, in contrast to 6.0% with cagrilintide 2.4 mg, 16.2% with semaglutide 2.4 mg, and just 0.9% with placebo.
When employing the treatment policy estimand2 approach, participants on CagriSema attained an impressive weight reduction of 20.4%, which was superior to the 11.5% reduction seen with cagrilintide 2.4 mg, 14.9% with semaglutide 2.4 mg, and 3.0% with placebo.
Throughout the trial, CagriSema, cagrilintide 2.4 mg, and semaglutide 2.4 mg demonstrated a safe and well-tolerated safety profile. The most frequently observed adverse effects associated with CagriSema were gastrointestinal; however, these were predominantly mild to moderate and tended to decrease over time, which aligns with expectations for the GLP-1 receptor agonist class.
“We are encouraged by the weight loss outcomes associated with CagriSema, which show superiority over both semaglutide and cagrilintide in monotherapy in the REDEFINE 1 trial. This was noteworthy given that only 57% of the participants achieved the maximum CagriSema dose,” stated Martin Holst Lange, Executive Vice President for Development at Novo Nordisk. “With the knowledge gained from the REDEFINE 1 study, we aim to further investigate the potential for enhanced weight loss with CagriSema.”
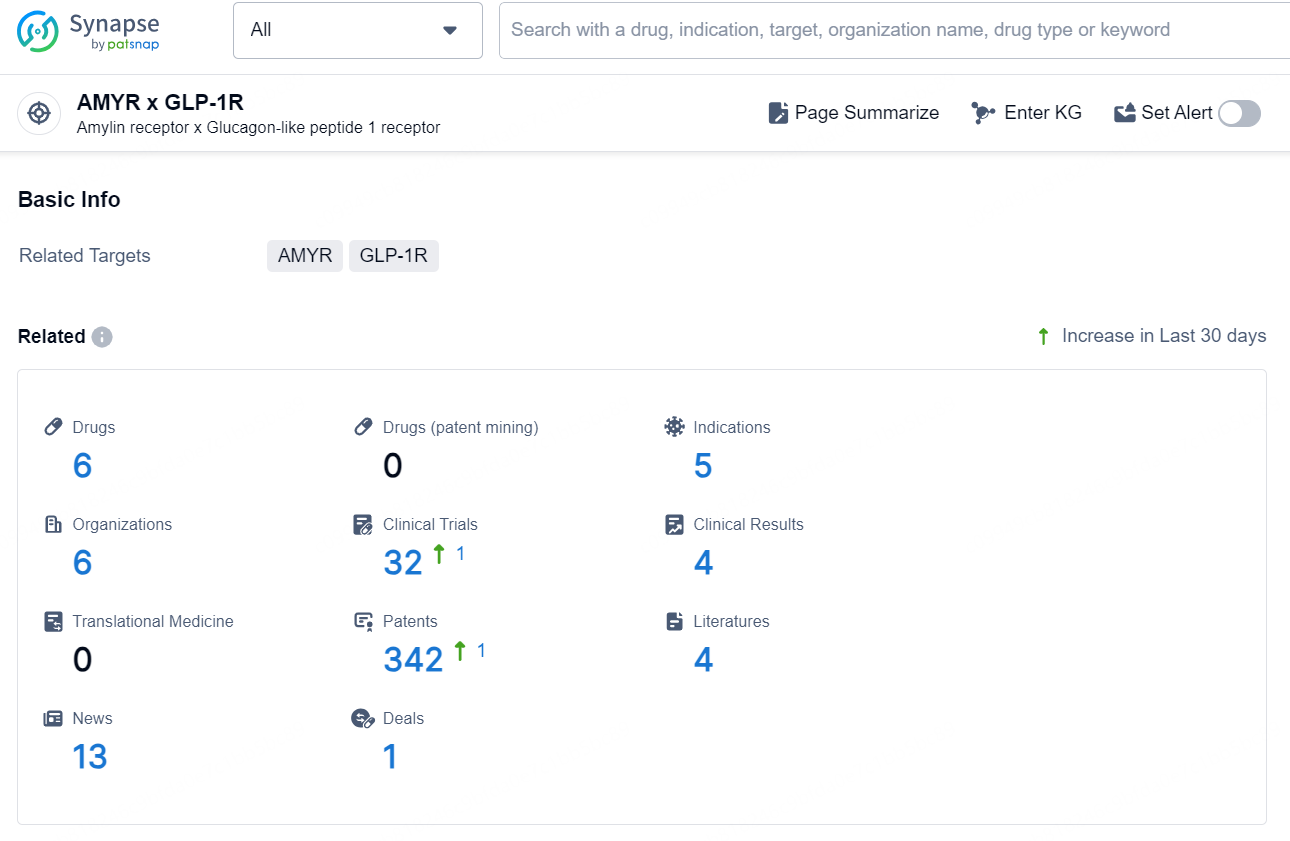
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 26, 2024, there are 6 investigational drugs for the AMYR x GLP-1R target, including 5 indications, 6 R&D institutions involved, with related clinical trials reaching 32 and as many as 342 patents.
CagriSema is a recombinant polypeptide drug that targets AMYR x GLP-1R and is being developed by Novo Nordisk A/S. The drug is primarily focused on therapeutic areas such as Endocrinology and Metabolic Disease, Digestive System Disorders, and Urogenital Diseases. The active indications for CagriSema include Diabetes Mellitus, Type 2, Obesity, Liver Diseases (Alcoholic), Chronic Kidney Diseases, and Overweight.