Vittoria Biotherapeutics Begins Phase 1 Trial Dosing for VIPER-101
Vittoria Biotherapeutics, Inc., a company focused on clinical-stage immunotherapy and the advancement of groundbreaking cell therapies for challenging medical conditions, announced that the first patient received dosing in its Phase 1 clinical trial of VIPER-101 in November. VIPER-101 is an autologous cell therapy designed to target relapsed and/or refractory (r/r) T cell lymphoma, a serious condition with few available treatment alternatives.
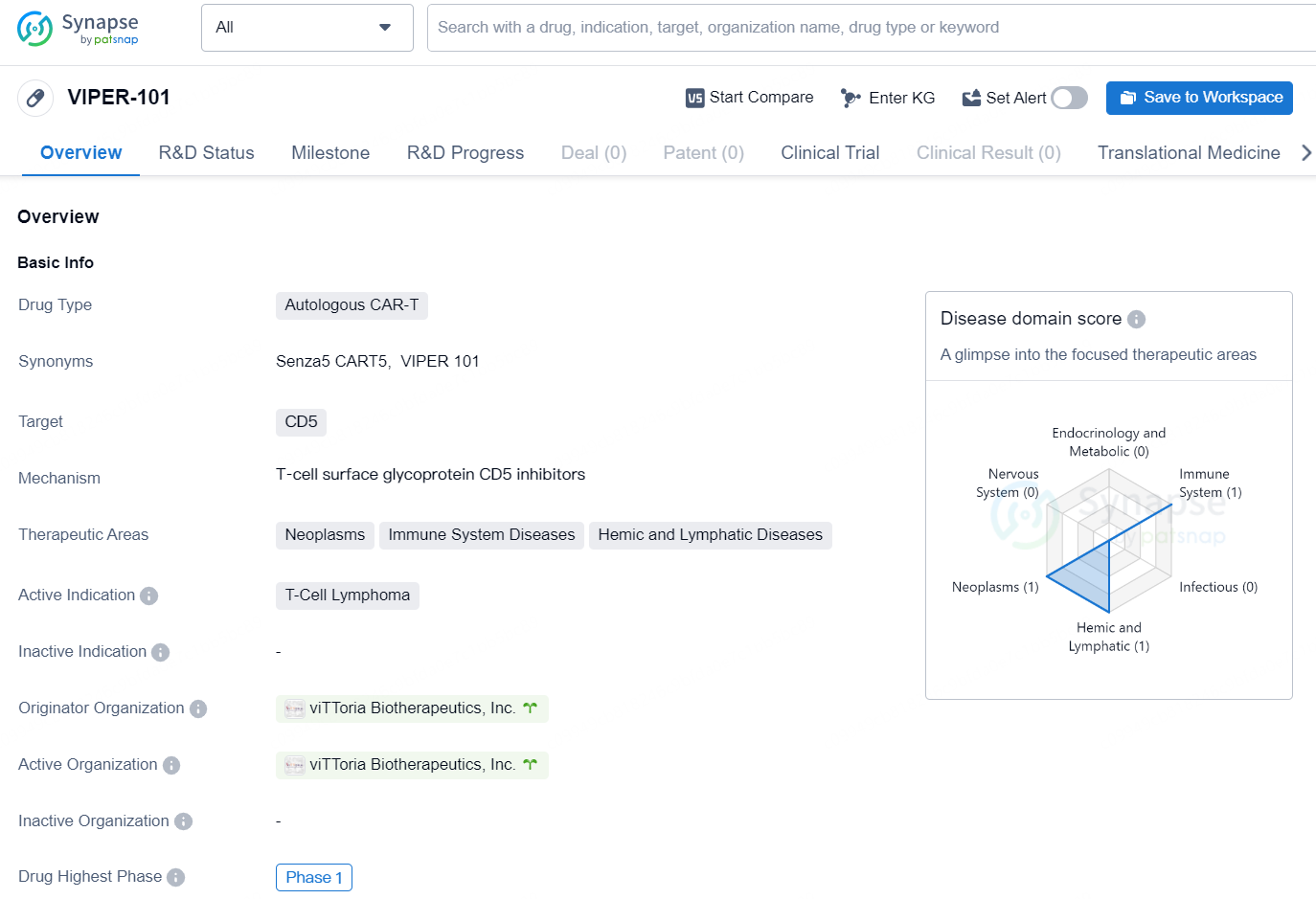
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
VIPER-101 utilizes Vittoria’s unique Senza5™ platform, which combines CD5 gene-editing technology with a swift five-day manufacturing process to provide a targeted and effective treatment for relapsed/refractory T cell lymphoma. This groundbreaking strategy aims to improve both the effectiveness and safety of traditional autologous cell therapies, offering renewed hope for patients whose cancers have not responded to standard treatments.
"This achievement marks a significant advancement in our goal of creating transformative therapies for patients facing challenging diseases," stated Nicholas Siciliano, Ph.D., CEO of Vittoria Biotherapeutics. "By tackling the major limitations of current CAR-T therapies, VIPER-101 is specifically designed to boost treatment potency, durability, and safety. We are excited to assess its therapeutic potential and anticipate producing significant data that may reshape the treatment approach for those fighting T cell lymphoma."
Marco Ruella, M.D., Scientific Founder of Vittoria Biotherapeutics and a Physician-Scientist at the University of Pennsylvania's Perelman School of Medicine, noted, "Even with progress in cell therapy, patients with T cell lymphoma still have very few treatment options available. Introducing this innovative method into clinical settings is the result of years of research dedicated to fulfilling the urgent need for new therapies for these patients."
The open-label Phase 1 trial aims to evaluate the safety and initial effectiveness of VIPER-101, as well as to establish the recommended Phase 2 dosage (RP2D) for patients with relapsed/refractory CD5 positive nodal non-Hodgkin lymphoma (NHL). The study is actively enrolling participants, and further details are available on ClinicalTrials.gov (NCT06420089).
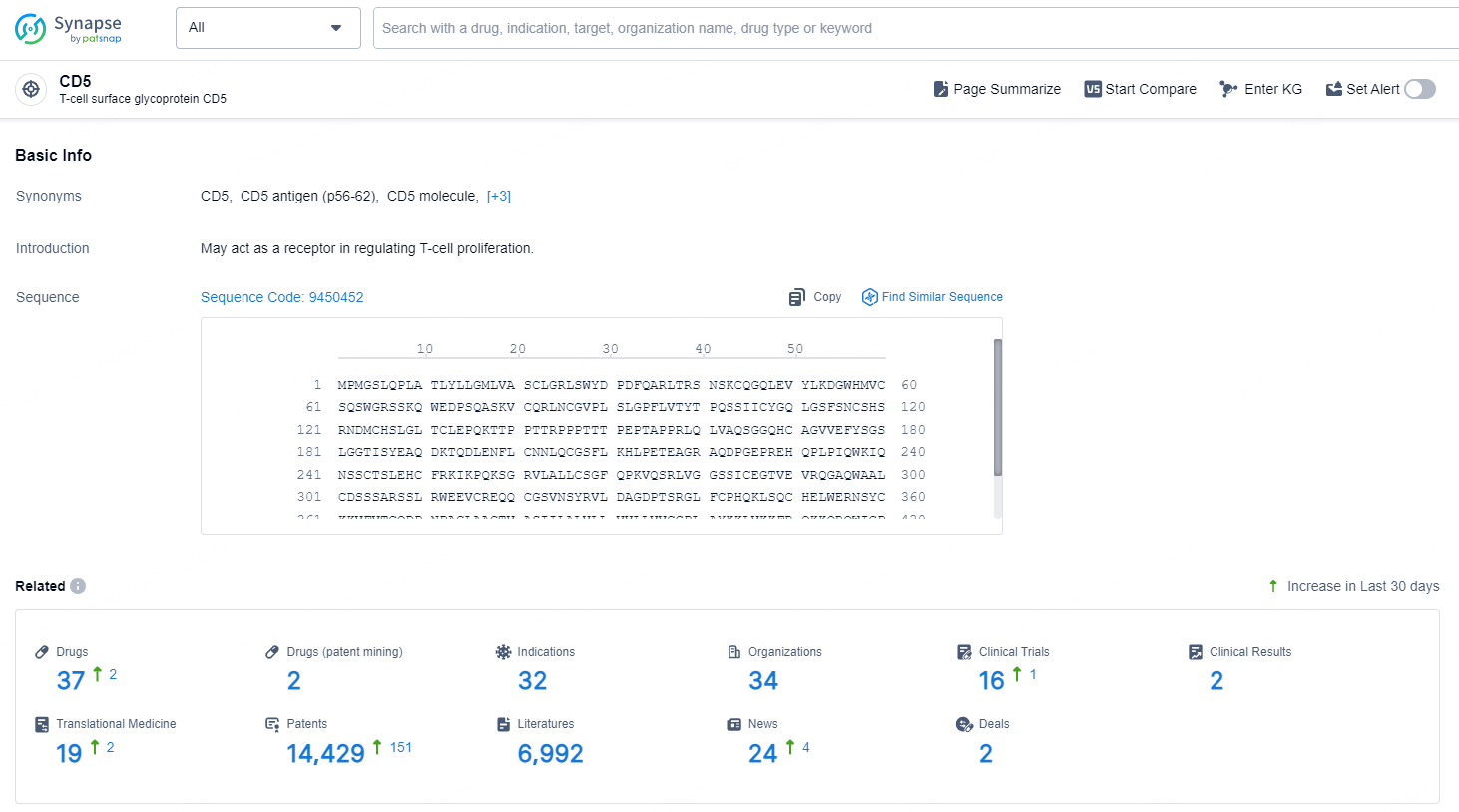
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 24, 2024, there are 37 investigational drugs for the CD5 target, including 32 indications, 34 R&D institutions involved, with related clinical trials reaching 16, and as many as 14429 patents.
VIPER-101 is an autologous CAR-T therapy developed by viTToria Biotherapeutics, Inc. The drug is designed to target CD5 and is primarily intended for the treatment of T-Cell Lymphoma. The therapeutic areas for which VIPER-101 is being developed include neoplasms, immune system diseases, and hemic and lymphatic diseases. The drug is currently in the Phase 1 stage of development, which is the highest phase it has reached globally.