A New Medication for Epileptic Encephalopathy Initiates Phase 3 Clinical Trials
Longboard Pharmaceuticals, a clinical-stage biopharmaceutical company dedicated to developing innovative medications for neurological disorders, has recently announced the initiation of the global Phase 3 DEEp SEA study. This study is designed to evaluate its investigational drug, bexicaserin, for the treatment of seizures in patients aged two and older with Dravet syndrome.
Developmental and epileptic encephalopathy syndromes
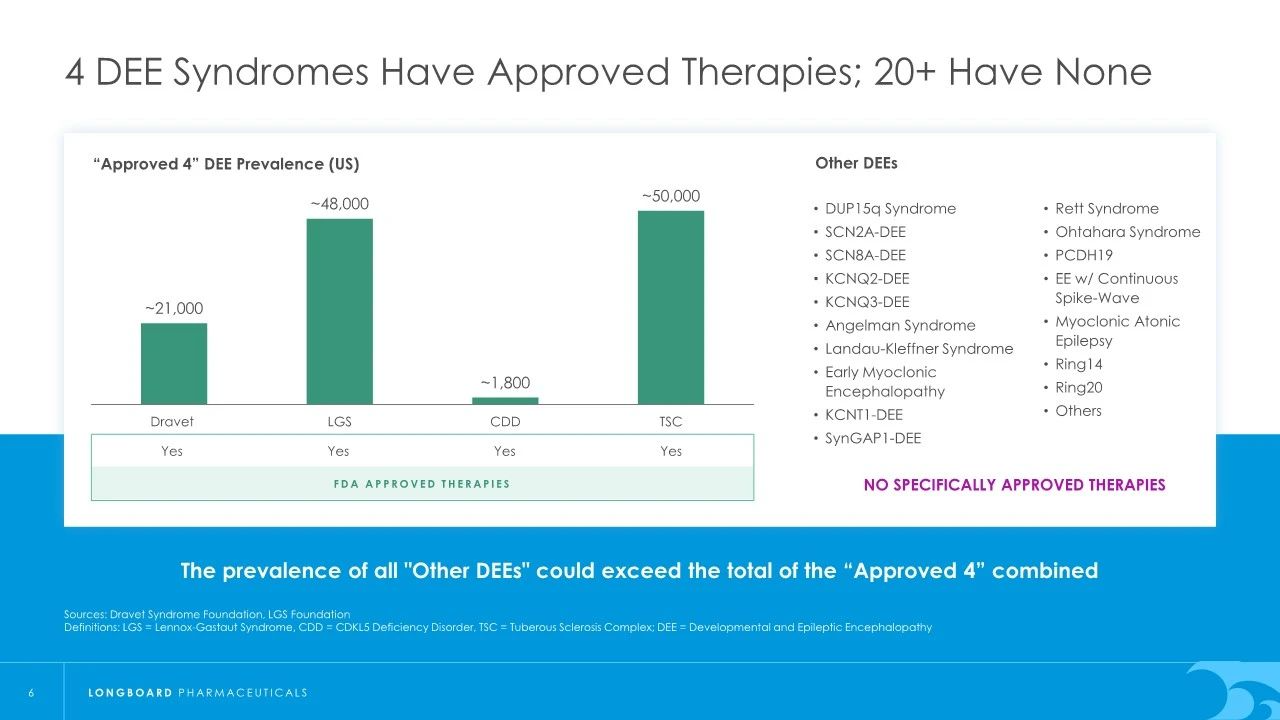
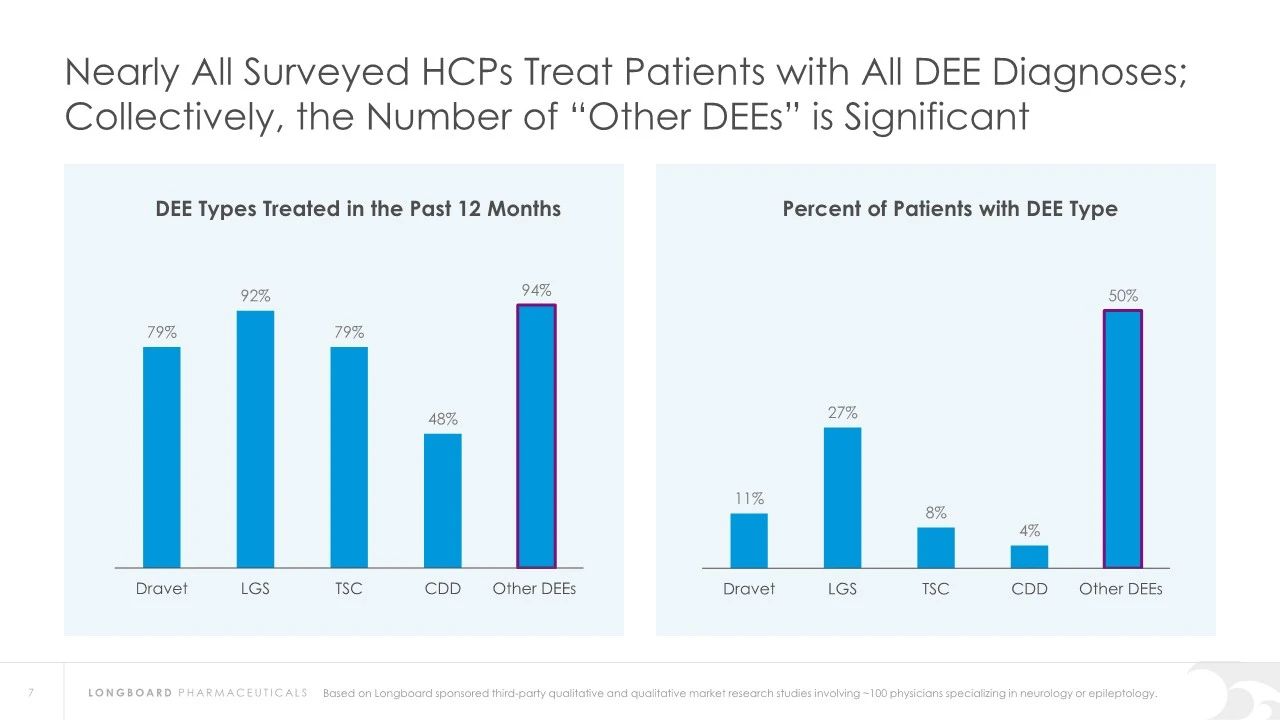
Developmental and epileptic encephalopathy syndromes (DEEs) are a group of rare neurological disorders that typically begin in childhood, affecting brain development and function. These conditions often result in multiple types of seizures, cognitive impairment, motor dysfunction, and behavioral issues. DEEs represent a heterogeneous group of diseases, each with specific genetic causes, clinical presentations, and treatment needs. There is a significant unmet need for treatments for epilepsy-related encephalopathies.
About Bexicaserin
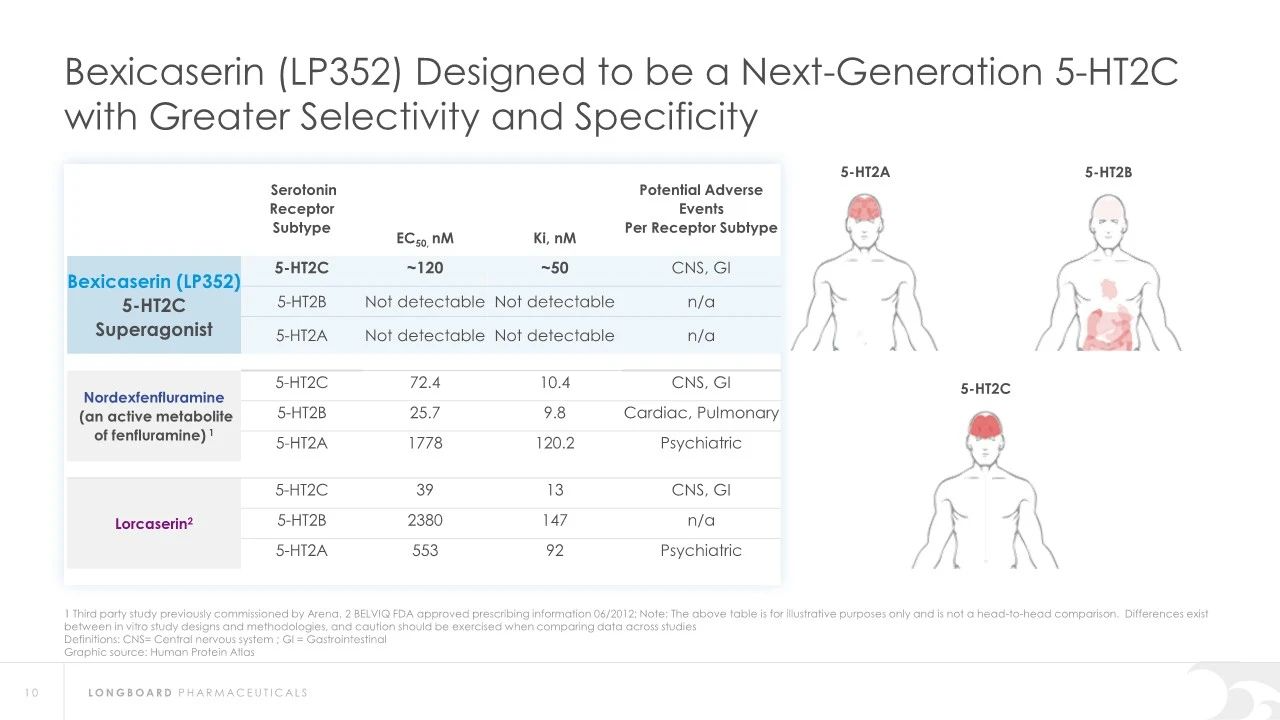
Bexicaserin (LP352), developed by Longboard Pharmaceuticals, is a novel 5-HT2C receptor superagonist with strong selectivity for 5-HT2A and 5-HT2B receptors. It is an oral medication related to the treatment of various CNS disorders, including obesity, schizophrenia, and substance addiction. Bexicaserin is currently undergoing Phase 3 clinical trials globally to assess its potential in treating seizures associated with developmental and epileptic encephalopathies (DEEs).
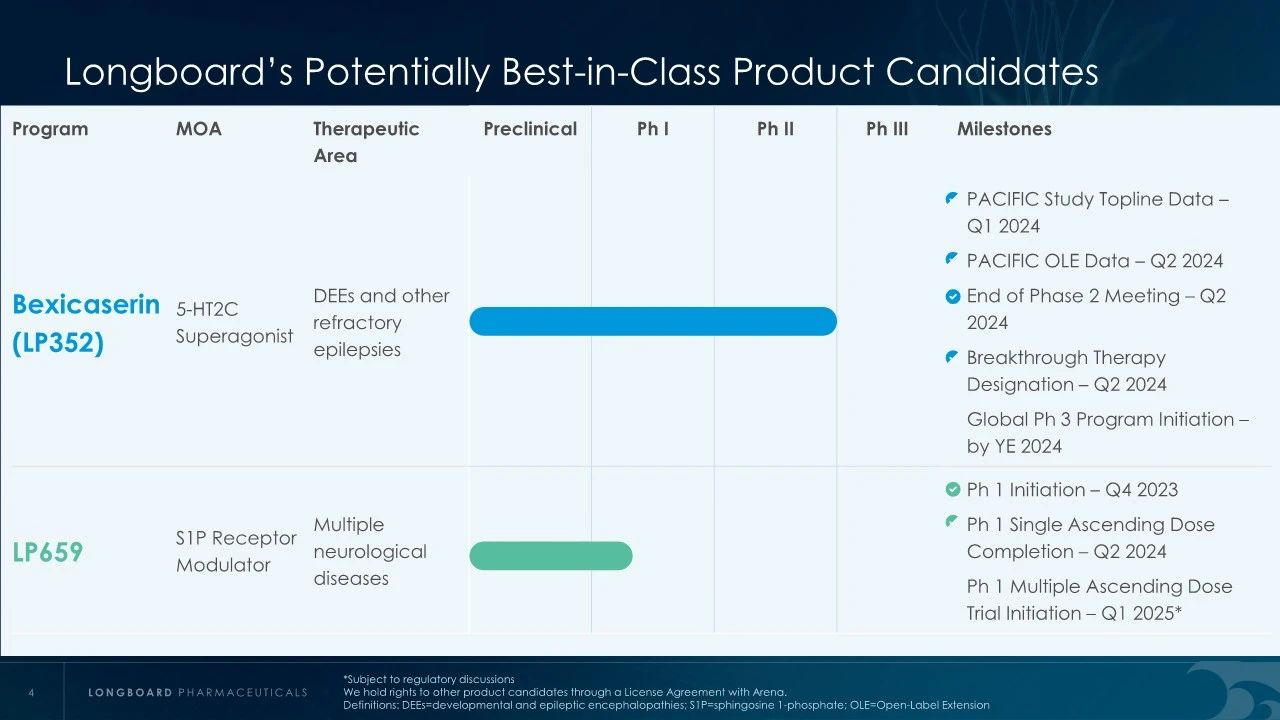
In earlier clinical studies, Bexicaserin has demonstrated a significant reduction in seizure frequency in patients with Dravet Syndrome (DS), Lennox-Gastaut Syndrome (LGS), and other types of DEEs. Specifically, in Dravet syndrome patients, Bexicaserin reduced seizure frequency by 72.1%, in LGS patients by 48.1%, and in patients with other types of DEEs by 61.2%.

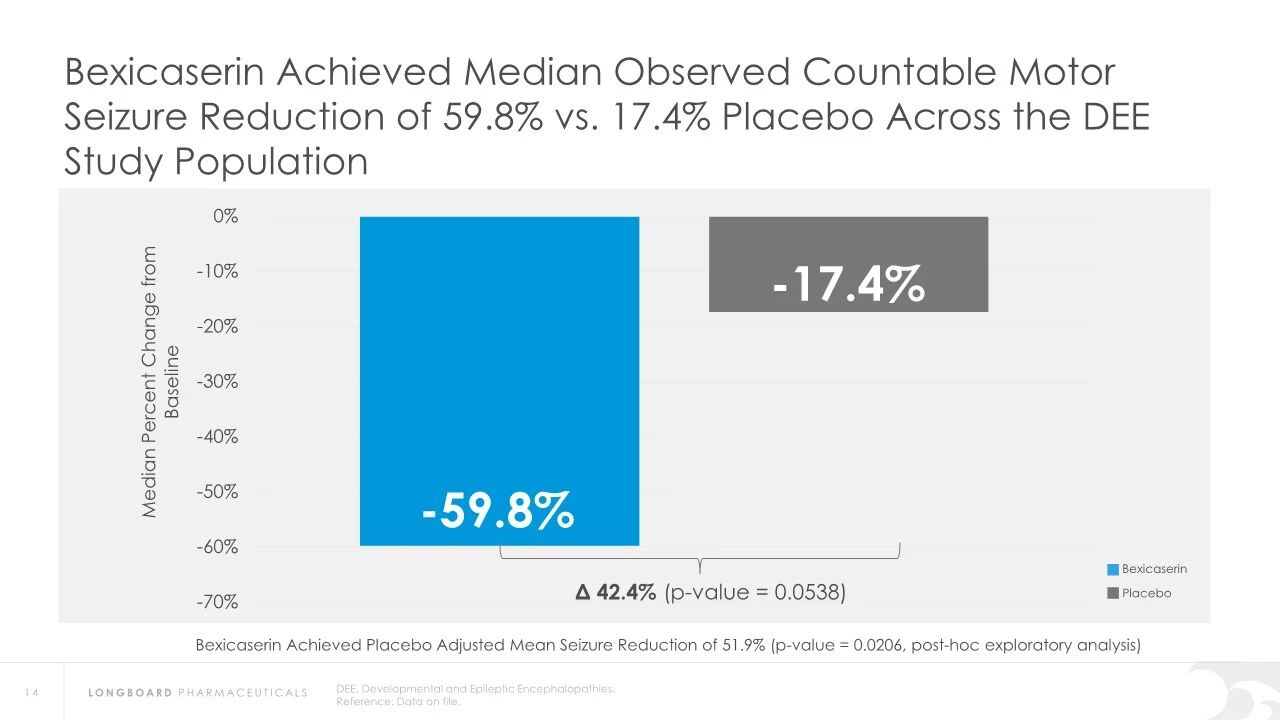
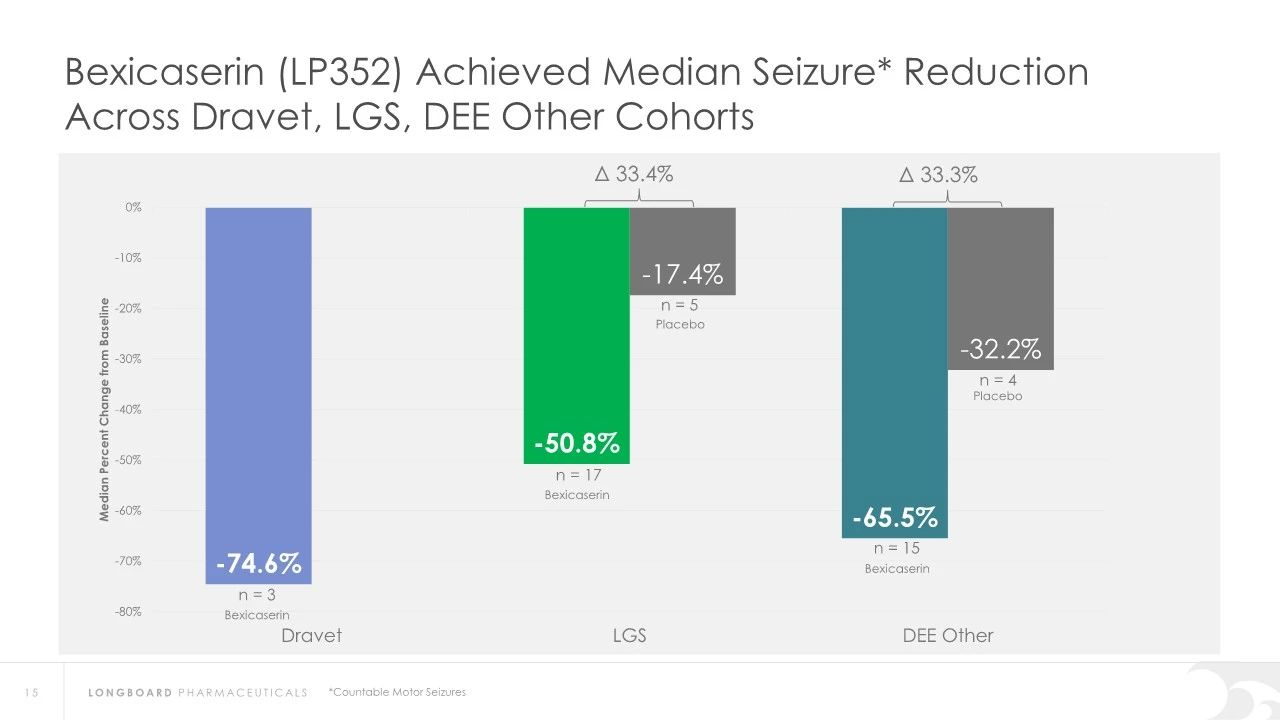
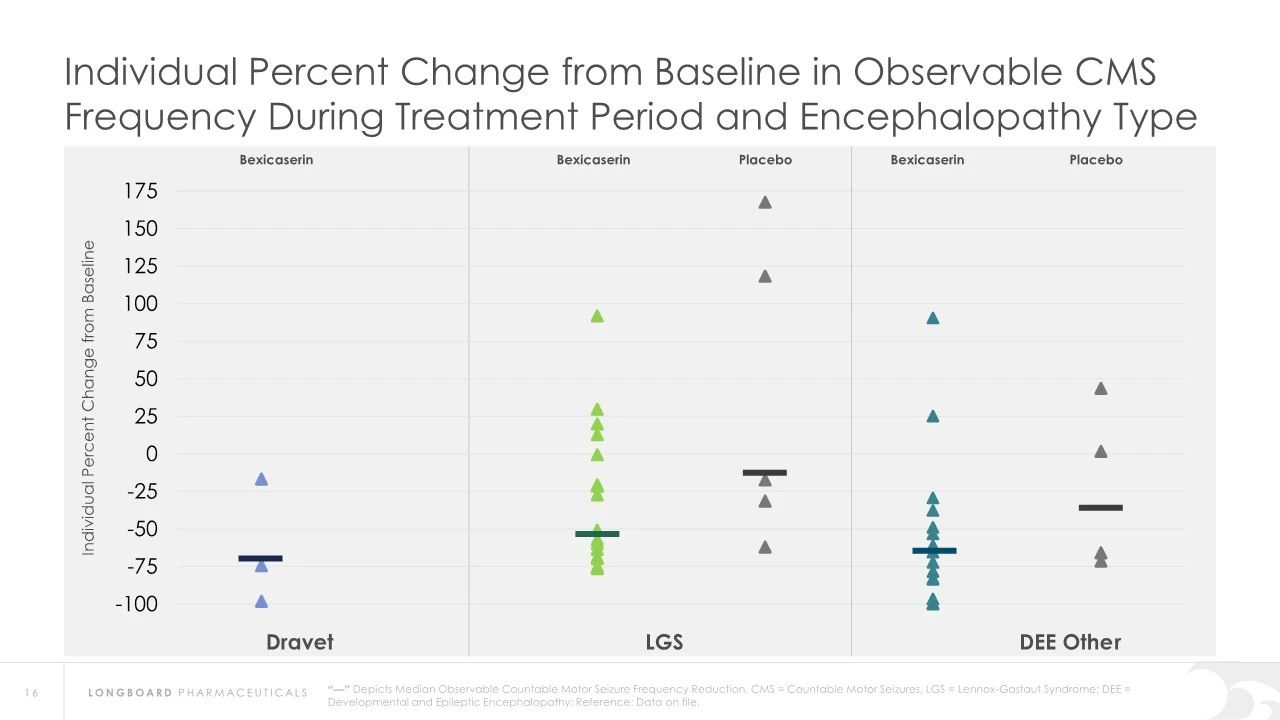
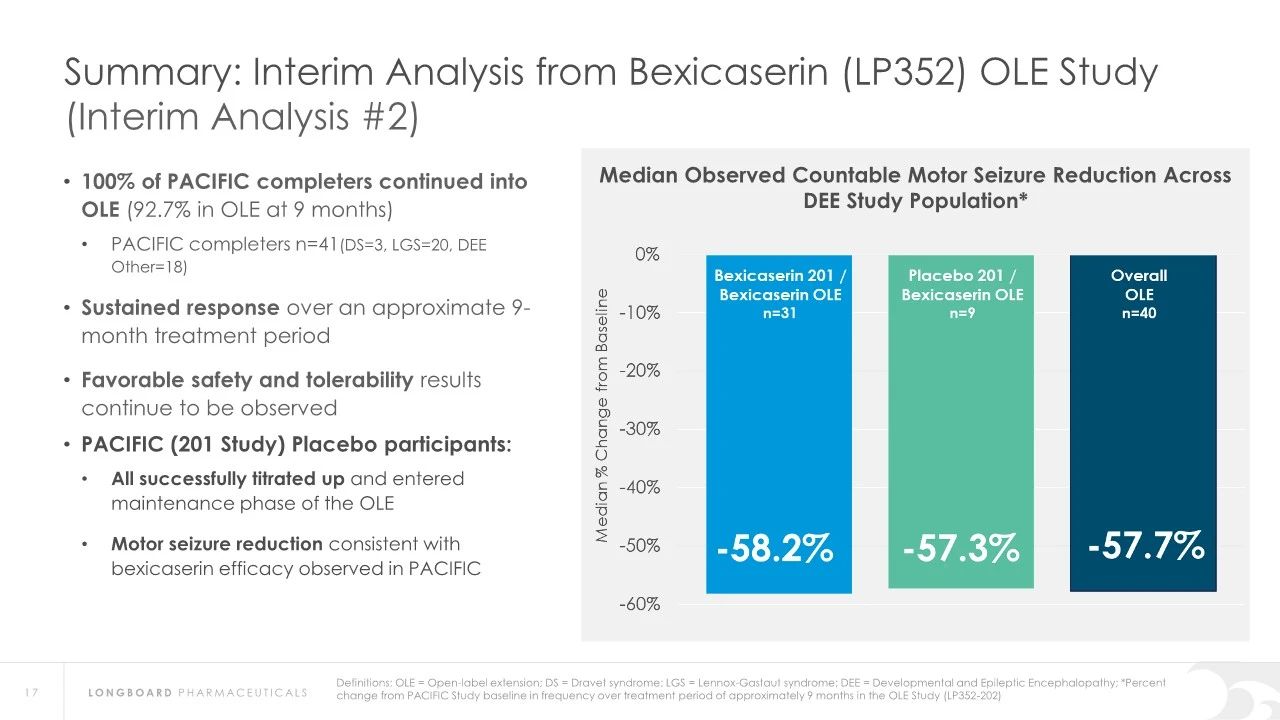
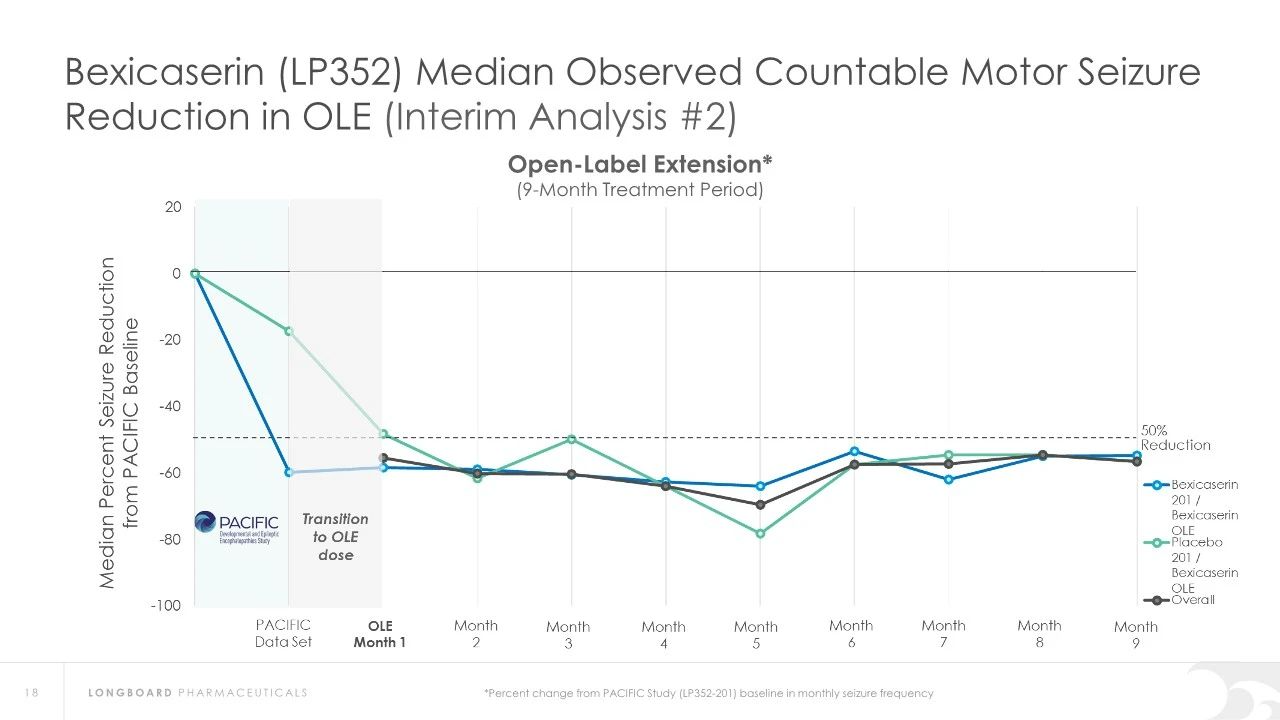
Regarding safety, Bexicaserin has shown good safety and tolerability in clinical trials. The U.S. Food and Drug Administration (FDA) has granted Bexicaserin the designation of a breakthrough therapy for treating seizures associated with DEEs, applicable to patients aged two and older.
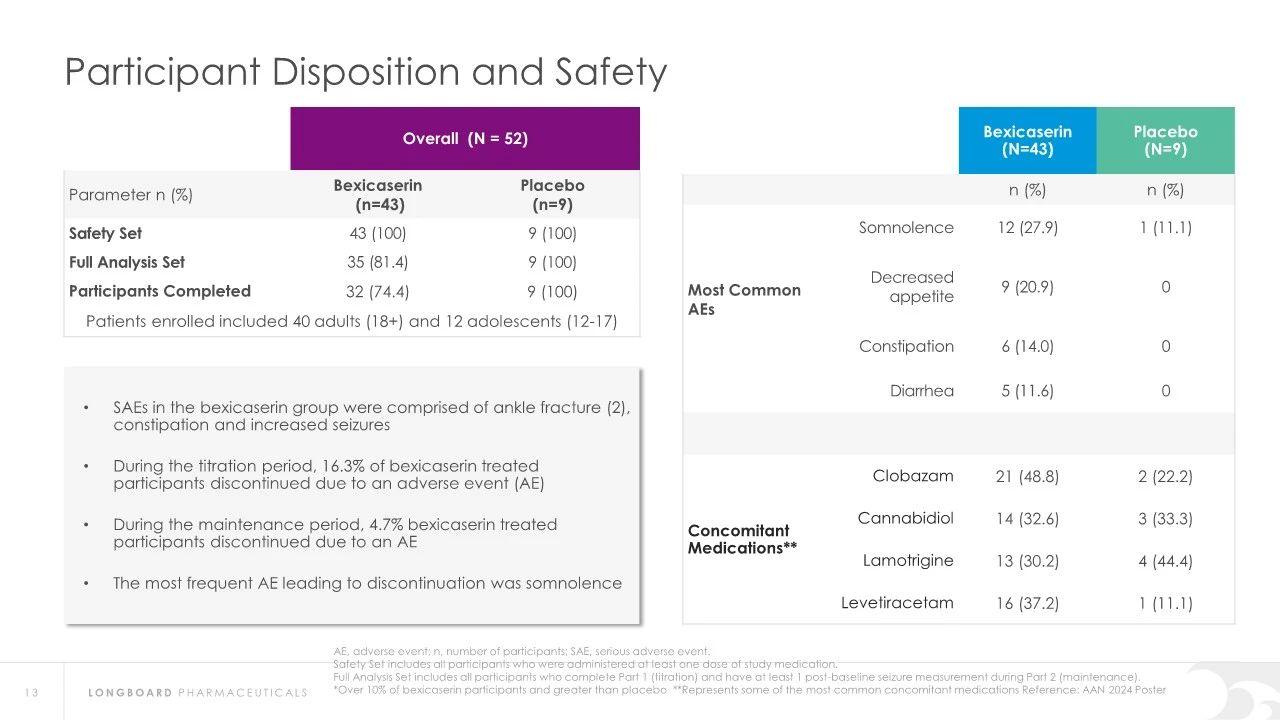
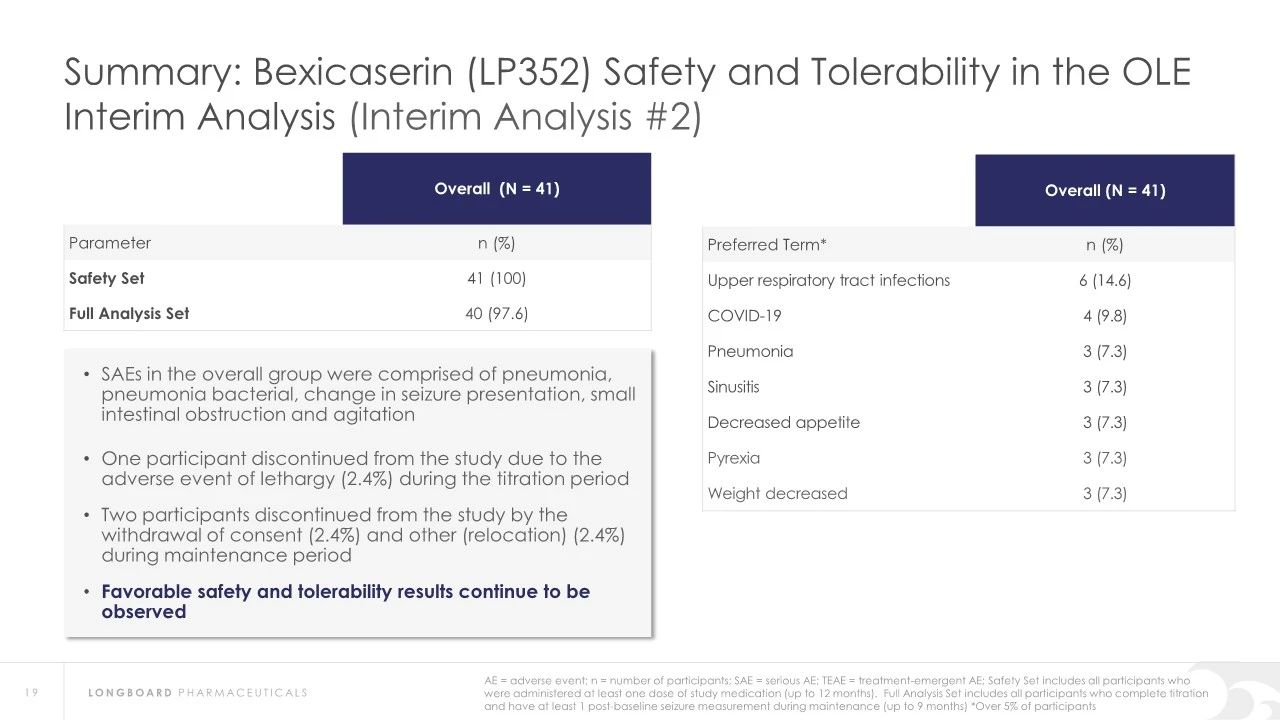
About DEEp SEA study
The ongoing DEEp SEA study (LP352-302) is a global, Phase 3, double-blind, placebo-controlled clinical trial aiming to evaluate the efficacy of Bexicaserin in treating seizures related to Dravet syndrome. Approximately 160 participants aged between two and 65 years will be involved.
An important secondary objective of the study is to assess the safety and tolerability of Bexicaserin. Following a five-week screening period and baseline assessment, participants will enter a three-week dose titration period, followed by a 12-week maintenance period at the highest tolerated dose. After the maintenance period, eligible participants may enroll in a 52-week DEEp open-label extension study (DEEp OLE study LP352-303).
The Phase 3 DEEp SEA study is part of the broader DEEp program, which will take place across approximately 80 global sites and involve around 480 participants with a range of developmental and epileptic encephalopathies (DEEs).


The U.S. FDA has granted Bexicaserin the designations of a rare pediatric disease and orphan drug for the treatment of Dravet syndrome. Additionally, the FDA has awarded breakthrough therapy status for Bexicaserin in treating DEEs-related seizures in patients aged two and older.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!