Skyhawk Therapeutics Presents Promising Preclinical Results for SKY-1214 at ENA Symposium
Skyhawk Therapeutics, Inc., a biotechnology firm in the clinical development phase, focuses on creating innovative small molecule therapies aimed at targeting essential RNA components. The Company has revealed striking preclinical findings showcasing the therapeutic promise of its proprietary compound, SKY-1214, during the European Organization for Research and Treatment of Cancer (EORTC)-National Cancer Institute (NCI)-American Association for Cancer Research (AACR) Molecular Targets and Cancer Therapeutics Symposium, commonly referred to as the "Triple Meeting."
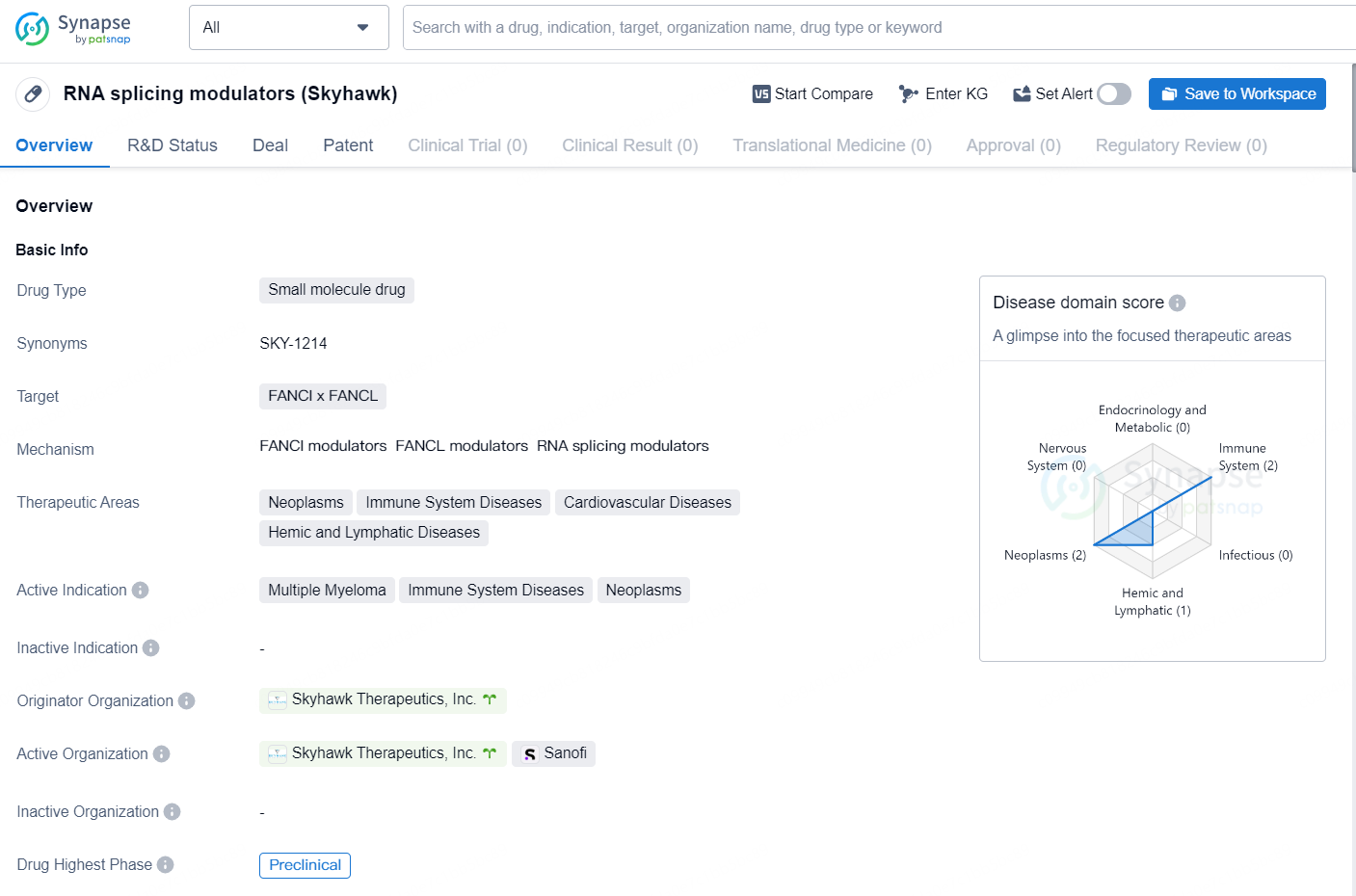
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
SKY-1214 is an innovative oral RNA splicing modulator that was identified via the company’s proprietary RNA-splicing technology. It is currently being investigated for challenging cases of multiple myeloma (MM) and non-Hodgkin’s lymphoma (NHL). This compound specifically targets FANCL/FANCI, which are vital elements of the Fanconi anemia DNA damage response pathway, utilized by MM and NHL cells to preserve their genomic stability.
According to the study's authors, SKY-1214 exhibits strong and effective anti-cancer properties in vitro across MM and NHL models, even in those with high-risk chromosomal and/or genetic changes (e.g., t(14;16), t(4;14), 1q+, and double-hit lymphoma). Moreover, administering SKY-1214 as a standalone treatment in vivo led to tumor suppression and complete regression to non-detectable levels at tolerable doses in xenograft mouse models of NHL and challenging MM, such as KMS-28BM.
Sergey Paushkin, M.D., Ph.D., Co-founder and Head of R&D at Skyhawk, expressed enthusiasm about the data and the impending IND submission for SKY-1214, which stands as the company’s most advanced oncology initiative. He noted that while there are numerous existing therapies for MM and NHL, every MM patient ultimately experiences disease progression, and only 50% of individuals with specific NHL subtypes achieve a cure. This situation highlights the critical need for new therapies that operate through unique mechanisms. The significant anti-cancer effects of SKY-1214, including in high-risk tumor cell lines, advocate for its continued development both as a monotherapy and in combination with other treatments for these challenging cancers.
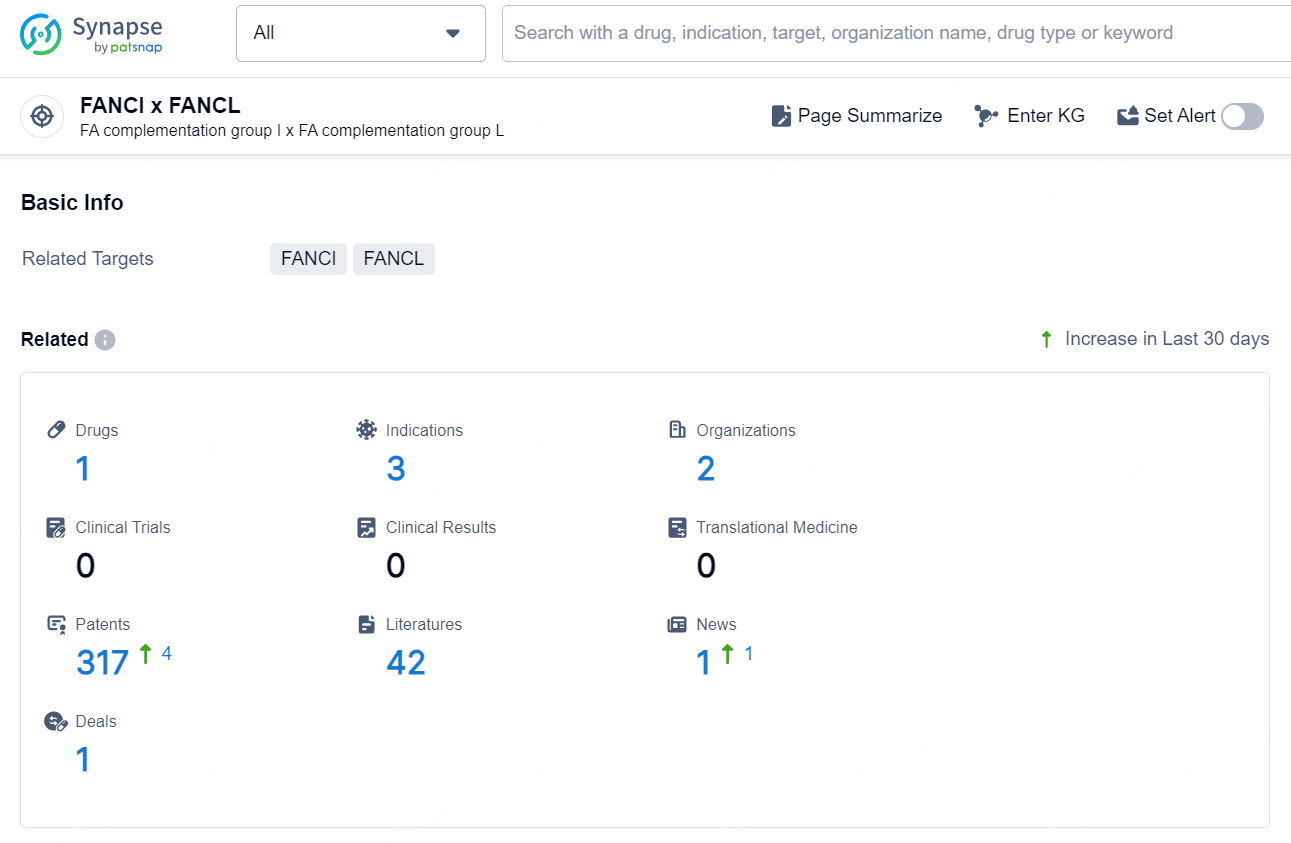
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 29, 2024, there are 1 investigational drug for the FANCI x FANCL target, including 3 indications, 2 R&D institutions involved, and as many as 317 patents.
The drug RNA splicing modulators, also known as Skyhawk, is a small molecule drug developed by Skyhawk Therapeutics, Inc. It targets the FANCI x FANCL genes and is currently in the preclinical phase of development. The therapeutic areas of focus for this drug include neoplasms, immune system diseases, cardiovascular diseases, and hemic and lymphatic diseases. The active indications for Skyhawk are multiple myeloma, immune system diseases, and neoplasms.