Novo Nordisk Completes Phase 2a Clinical Study of New Weight Loss Product
On September 20, Novo Nordisk announced on its official website the primary results of a Phase 2a clinical trial for its investigational drug monlunabant (previously known as INV-202). While the drug demonstrated significant weight loss effects, the mild to moderate neuropsychiatric side effects, specifically anxiety, irritability, and sleep disturbances, have garnered more attention from the market.
Monlunabant is a small molecule oral CB1 receptor inverse agonist, intended for the treatment of obesity and metabolic syndrome. This drug was acquired by Novo Nordisk through the purchase of Inversago Pharmaceuticals in August 2023.
Inversago, a Canadian biotechnology start-up, has been focusing on developing inverse agonist products for the CB1 receptor. The company is conducting multiple clinical developments for several CB1 receptor-targeted inhibitors, with indications including obesity, diabetic nephropathy, and pulmonary fibrosis.

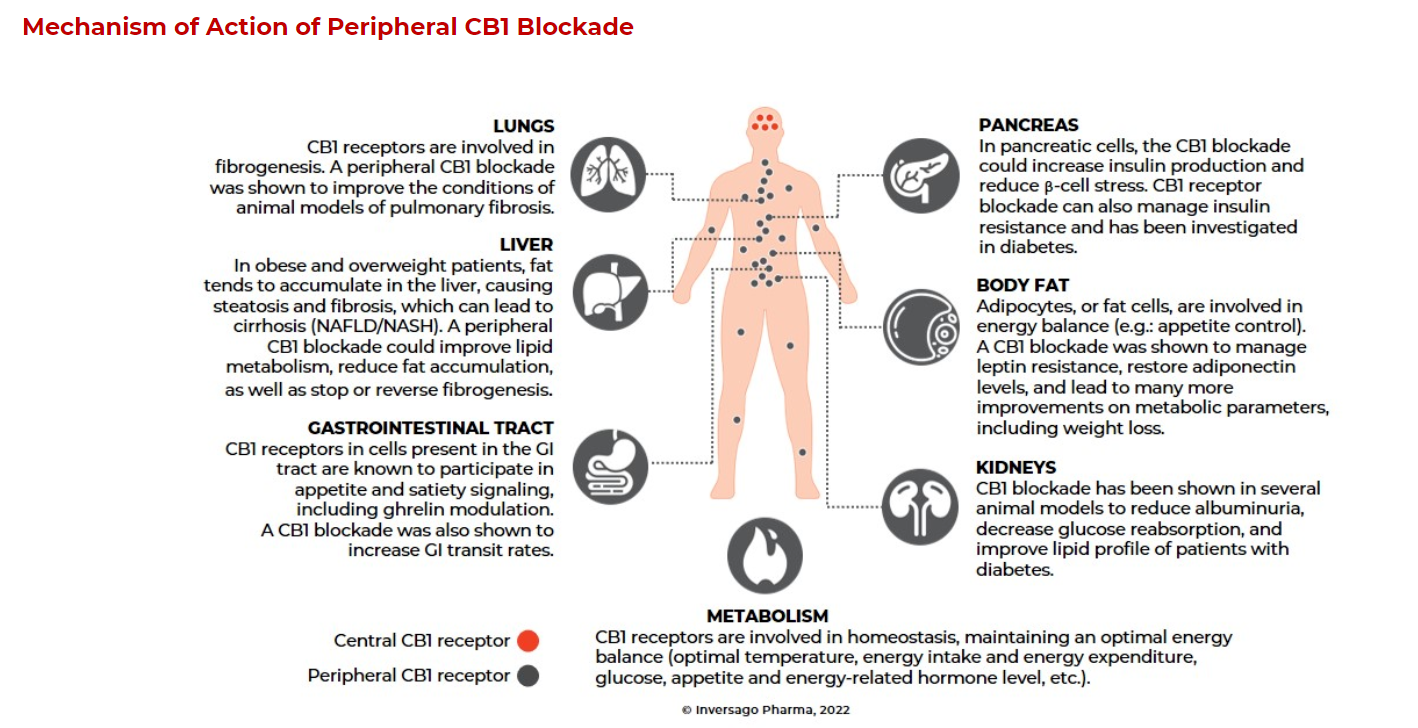
Monlunabant (INV-202) is a potentially first-in-class small molecule CB1 receptor (CB1R) inverse agonist being developed for the treatment of metabolic diseases. It is specifically designed to preferentially block CB1R in peripheral tissues such as kidneys, gastrointestinal tract, liver, pancreas, adipose tissue, muscle, and lungs. Peripheral CB1R blockade is well-documented for its therapeutic effects in a range of cardiometabolic and fibrotic diseases, paving the way for potential treatments for a vast number of patients with unmet needs. Based on positive results from phase 1 clinical trials, Inversago initiated a phase 2 clinical trial in obese subjects. Additionally, the company has multiple products in development, including the INV-300 series, INV-347, and INV-101.

In this recent update involving 243 patients with obesity and metabolic syndrome, a 10mg daily dose of monlunabant resulted in an average weight loss of 7.1 kg after 16 weeks, compared to a 0.7 kg loss in the placebo group. However, higher doses offered limited additional weight loss benefits. In terms of safety, the most common adverse events were gastrointestinal reactions, mostly mild to moderate and dose-related. Compared to the placebo, patients using monlunabant more frequently reported mild to moderate neuropsychiatric side effects, primarily anxiety, irritability, and sleep disturbances, which were also dose-related. No serious adverse events associated with neuropsychiatric side effects were reported.
Despite monlunabant showing potential for weight reduction, Novo Nordisk executives believe further research is necessary to determine the optimal dosage that balances safety and efficacy. The company plans to launch a larger Phase 2b trial in 2025 to further explore the dosage and long-term safety of monlunabant in a global population. The market's response to these results has been mixed. Some analysts have questioned the commercial viability of monlunabant and similar drugs, expressing concerns over the side effects. Moreover, compared to Eli Lilly & Co.'s oral drug orforglipron, monlunabant's weight loss effectiveness in trials appears to be inferior. These concerns resulted in a roughly 5% drop in Novo Nordisk's stock price following the announcement.
Regarding Cannabinoid Receptors
Historically, cannabis has been used as a medicine, derived from plants of the genus Cannabis. Its main components are tetrahydrocannabinol (THC) and cannabidiol (CBD). Cannabinoids are a group of terpenophenolic compounds found in cannabis, and they naturally occur in the nervous and immune systems of animals.
In the 1980s, two G protein-coupled receptors specifically responsive to cannabinoids were discovered, known as cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2).
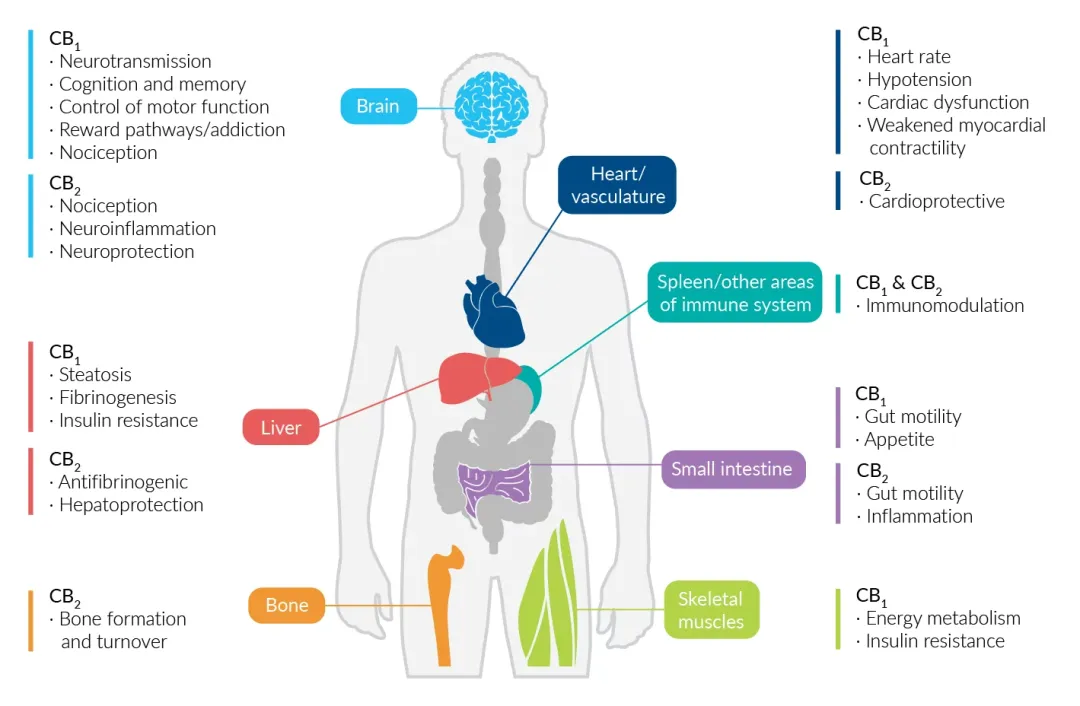
Cannabinoid receptors and endocannabinoids not only play a role in regulating many physiological functions of the central and peripheral nervous systems, but they are also involved in regulating physiological functions in various peripheral regions of the body, including the endocrine system, immune system, gastrointestinal tract, cardiovascular system, and reproductive system.
Although CB1 receptors are primarily located in the brain, they are also expressed in the spleen, lungs, thymus, heart, and blood vessels. Within the central nervous system, they are most abundant in the axons and presynaptic terminals of neurons in the amygdala, hippocampus, cortex, basal ganglia, and cerebellum. These receptors are closely associated with GABAergic (inhibitory) and glutamatergic (excitatory) neurons, mediating the sustained release of inhibitory and excitatory neurotransmitters.
CB2 receptors are most abundant in immunocompetent tissues outside the brain (such as the spleen, tonsils, bone marrow, pancreas, and peripheral blood leukocytes) and within the brain (astrocytes and microglia). They function in regulating the migration of immune cells and the release of cytokines and are also involved in the cardiovascular and respiratory systems, bones, gastrointestinal tract, liver, and reproductive system.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!