Is Ozanimod approved by the FDA?
Yes, Ozanimod is FDA approved. The U.S. Food and Drug Administration (FDA) approved Ozanimod, marketed under the brand name Zeposia, on March 25, 2020. Ozanimod belongs to the drug class of selective immunosuppressants.
What is Ozanimod?
Ozanimod is an oral medication used to treat relapsing forms of multiple sclerosis (MS) in adults, which includes clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease. Additionally, it is used to treat adults with moderately to severely active ulcerative colitis.
How Does Ozanimod Work?
Ozanimod works by modulating the immune system. It selectively targets and binds to sphingosine 1-phosphate receptors, which are involved in the movement of immune cells. By binding to these receptors, Ozanimod prevents the immune cells from reaching the central nervous system and gastrointestinal tract, thus reducing inflammation and the autoimmune response that contributes to the symptoms of multiple sclerosis and ulcerative colitis.
Dosage and Administration
Ozanimod is available in oral capsules and follows a specific dosing schedule to minimize side effects:
- Dose Titration Regimen:
- Days 1 through 4: 0.23 mg orally once a day.
- Days 5 through 7: 0.46 mg orally once daily.
- Day 8 and thereafter: 0.92 mg orally once a day.
- Maintenance Dose:
- 0.92 mg orally once daily starting on Day 8.
This titration schedule helps to reduce the risk of side effects associated with the initiation of Ozanimod therapy.
Warnings and Precautions
- Infections: Ozanimod can increase the risk of infections, including serious or fatal infections. This risk may persist for up to three months after discontinuation of the medication. Patients should report symptoms such as fever, flu-like symptoms, cough, rash, and frequent urination to their healthcare provider.
- Heart Conditions: Ozanimod can slow heart rate, especially when treatment is initiated. Patients with serious heart conditions such as AV block, sick sinus syndrome, recent heart failure, heart attack, or stroke should not use Ozanimod unless monitored by a healthcare professional.
- MAO Inhibitors: Patients should not use Ozanimod if they have taken an MAO inhibitor within the past 14 days due to the risk of serious drug interactions.
Side Effects
Common side effects of Ozanimod include:
- Headache
- Back pain
- Urination problems
- High or low blood pressure
- Abnormal liver function tests
- Cold symptoms (stuffy nose, sneezing, sore throat)
Serious side effects that require immediate medical attention include slow heartbeats, chest pain, shortness of breath, severe headache, vision changes, and symptoms of infection.
Drug Interactions
Ozanimod can interact with a variety of medications, including heart rhythm medications, antidepressants, and drugs that weaken the immune system. Patients should inform their healthcare provider about all medications they are taking to avoid potential interactions.
Conclusion
Ozanimod (Zeposia) is an FDA-approved medication for the treatment of relapsing forms of multiple sclerosis and moderately to severely active ulcerative colitis in adults. Approved on March 25, 2020, it offers a targeted approach to managing these conditions by modulating the immune system. Patients should follow the prescribed dosing schedule and consult their healthcare provider for monitoring and management of any potential side effects or drug interactions.
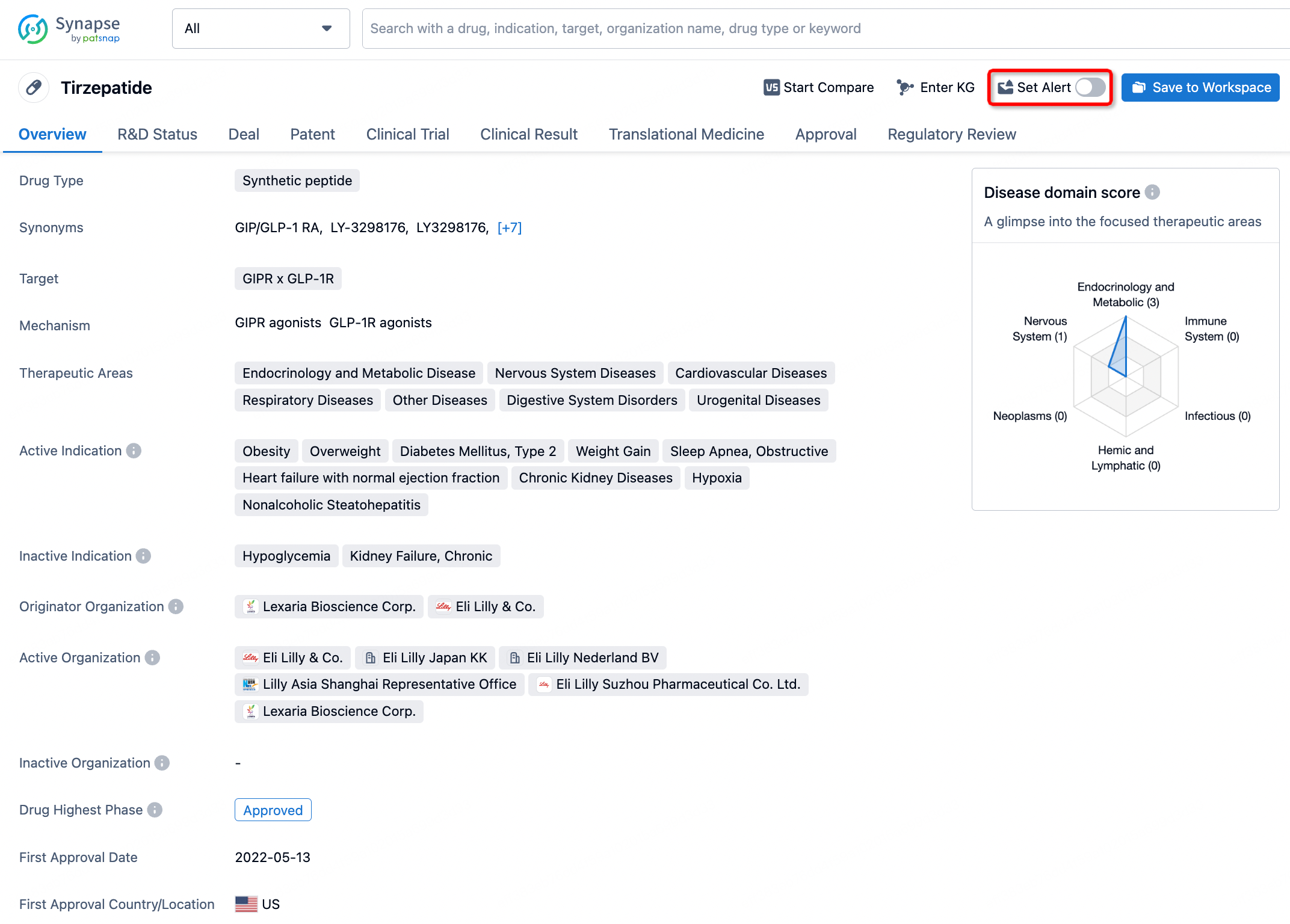
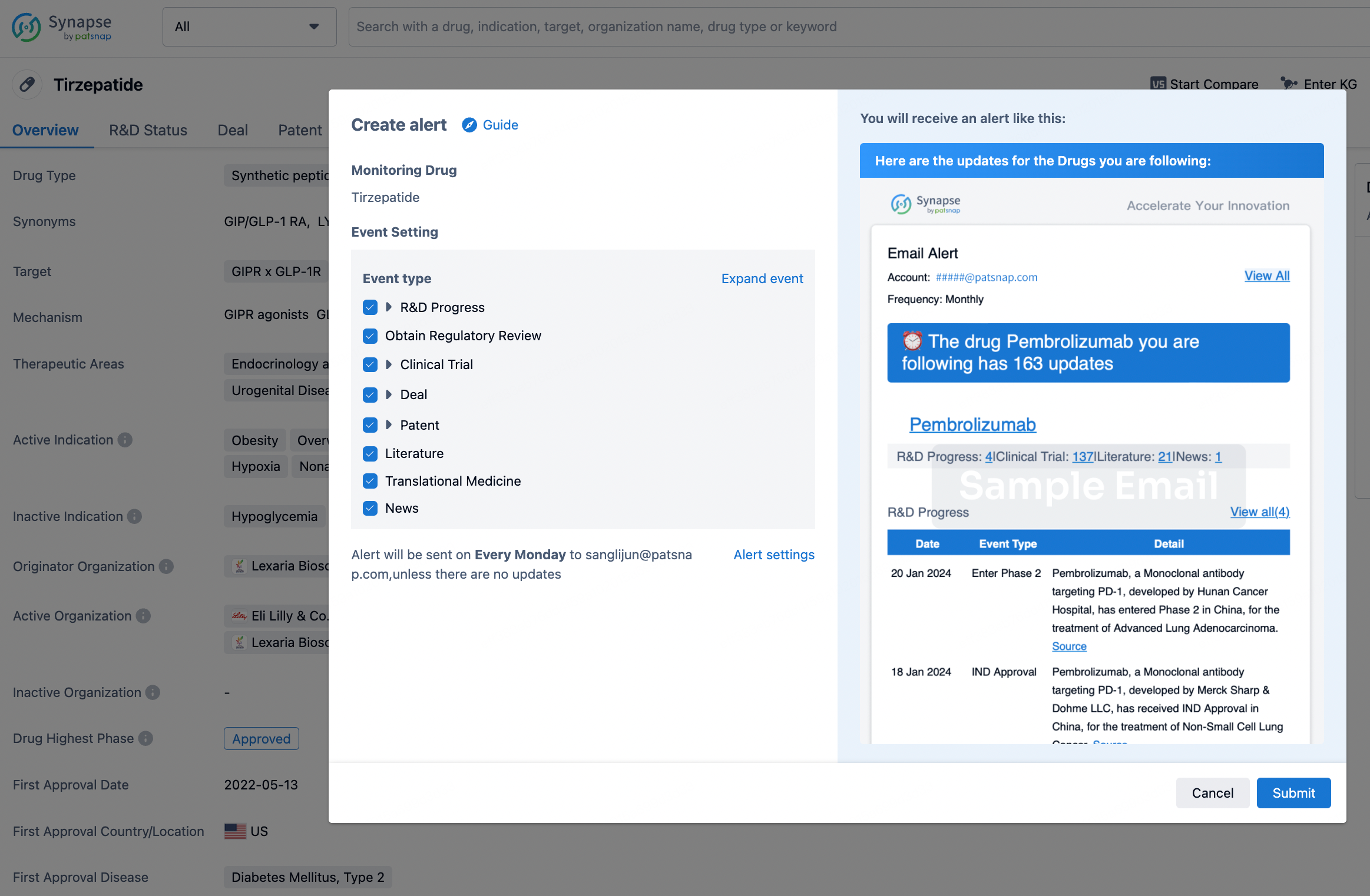
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!