Kezar Life Sciences Reports Clinical Hold on Zetomipzomib IND for Lupus Nephritis Treatment
Kezar Life Sciences, Inc. (Nasdaq: KZR), a biotechnology company in the clinical development stage, is creating a new small molecule to address unmet needs in immune-mediated diseases. The company announced that during a teleconference with the U.S. Food and Drug Administration (FDA), they were informed that the Investigational New Drug (IND) application for zetomipzomib, intended for lupus nephritis (LN) treatment, has been put on clinical hold. This decision was made after Kezar communicated to the FDA their voluntary suspension of both enrollment and dosing in the Phase 2b PALIZADE clinical trial involving zetomipzomib for patients with active LN, as recommended by the trial’s Independent Data Monitoring Committee (IDMC).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Following a review of new safety information, the IDMC made a suggestion that considered four Grade 5 (fatal) serious adverse events (SAEs) that transpired during the trial involving participants from the Philippines and Argentina. The FDA has informed Kezar that a formal clinical hold letter will be issued within a 30-day timeframe.
Chris Kirk, PhD, the CEO of Kezar, stated, "We remain deeply committed to ensuring patient safety and are focusing on a thorough investigation of these incidents as we strive to advance the zetomipzomib development program. Our current IND for zetomipzomib in autoimmune hepatitis treatment is not affected. The Phase 2a PORTOLA clinical trial for autoimmune hepatitis patients is ongoing, and no Grade 4 or 5 SAEs have been reported in this trial so far."
Lupus nephritis (LN) represents one of the most severe manifestations of systemic lupus erythematosus (SLE). It consists of various vascular, glomerular, and tubulointerstitial lesions and affects about half of SLE patients within a decade of diagnosis. LN contributes significantly to morbidity, increasing the likelihood of end-stage renal disease, which may necessitate dialysis or a kidney transplant, and also raises the risk of mortality. Treatment options for LN are limited, usually involving an initial induction therapy to accomplish remission, followed by prolonged maintenance therapy to avert relapse.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
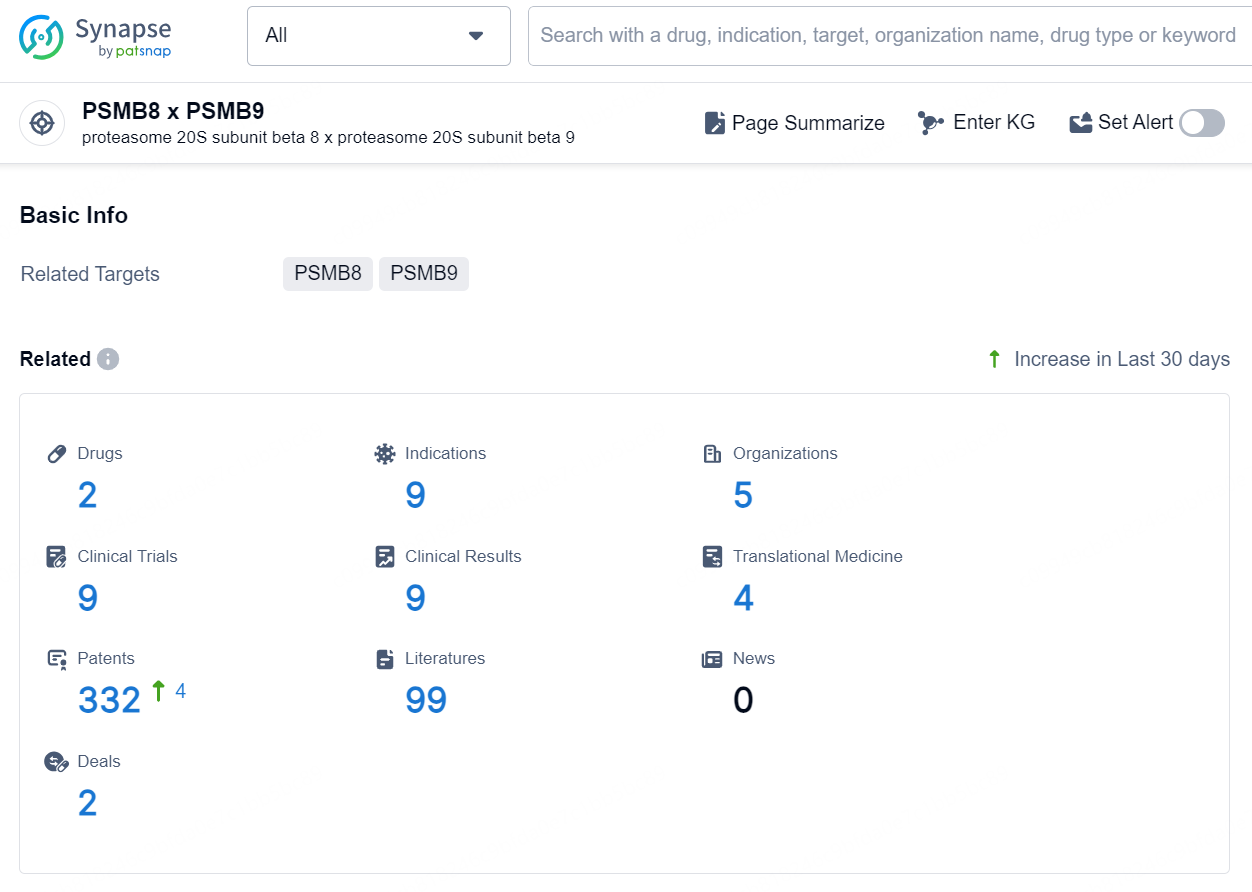
According to the data provided by the Synapse Database, As of October 9, 2024, there are 2 investigational drugs for the PSMB8 x PSMB9 targets, including 9 indications, 5 R&D institutions involved, with related clinical trials reaching 9, and as many as 332 patents.
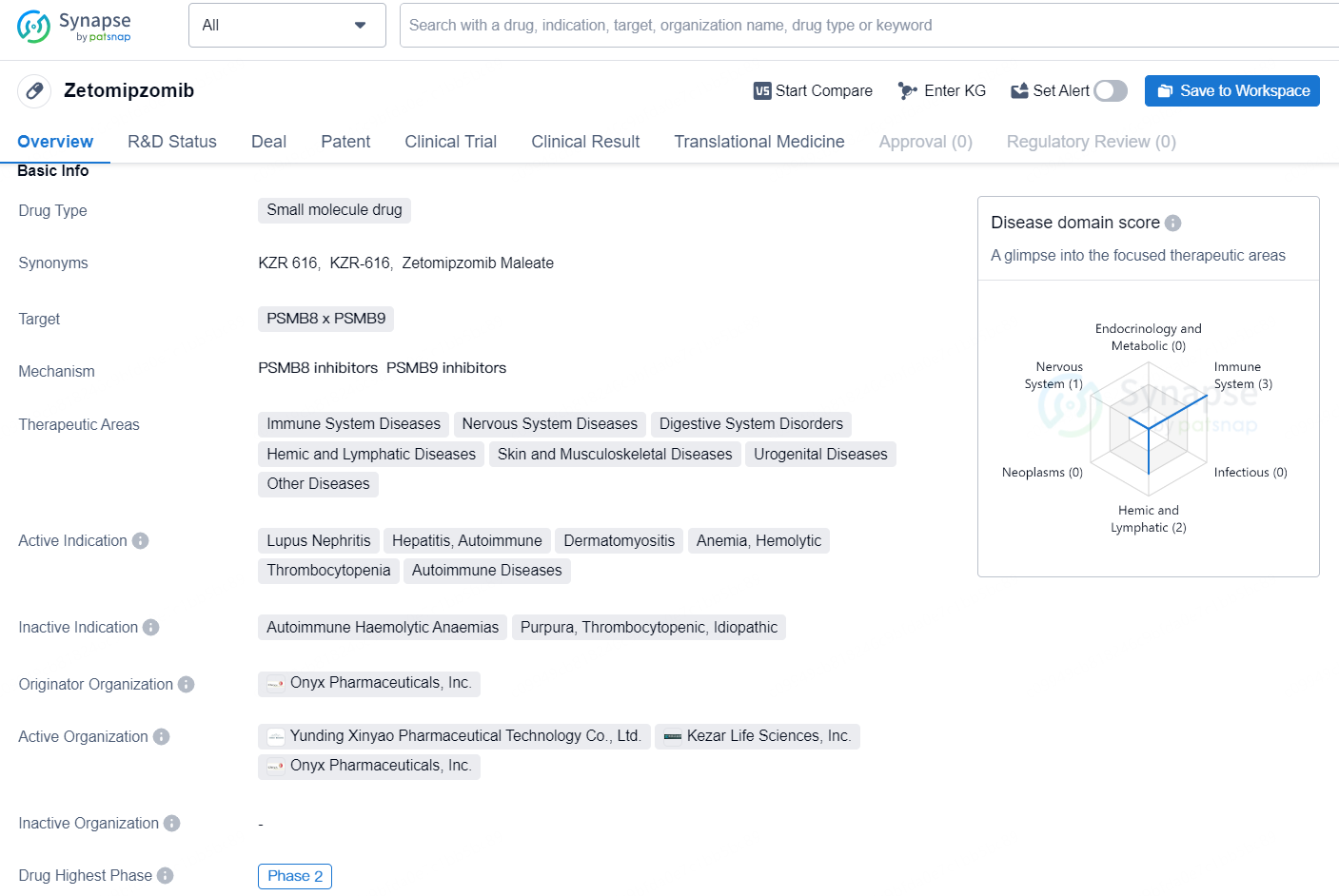
Zetomipzomib is a small molecule drug that targets PSMB8 x PSMB9 and is being developed by Onyx Pharmaceuticals, Inc. The drug is being studied for its potential in treating a wide range of therapeutic areas, including immune system diseases, nervous system diseases, digestive system disorders, hemic and lymphatic diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases.