LYNPARZA® Boosts Survival in Early Breast Cancer: OlympiA Phase III Trial
Recent findings from the OlympiA Phase III clinical trial indicate that LYNPARZA®(olaparib), developed by AstraZeneca in collaboration with Merck & Co., Inc. (known as MSD outside the US and Canada), exhibited significant and lasting enhancements in overall survival (OS), invasive disease-free survival (IDFS), and distant disease-free survival (DDFS) after six years for individuals with germline BRCA-mutated (gBRCAm) HER2-negative high-risk early-stage breast cancer.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The findings were unveiled today at the San Antonio Breast Cancer Symposium 2024 (SABCS) (#GS1-09) and build upon the favorable primary outcomes published in The New England Journal of Medicine.
In a median follow-up period of 6.1 years among eligible patients who had undergone local treatment and standard neoadjuvant or adjuvant chemotherapy, data indicated that LYNPARZA decreased the risk of mortality by 28% (hazard ratio [HR] 0.72; 95% confidence interval [CI] 0.56-0.93) when compared to placebo. Additionally, 87.5% of patients receiving LYNPARZA were alive at the end of the study, in contrast to 83.2% in the placebo group.
LYNPARZA further exhibited sustained and clinically meaningful enhancements in both the primary and secondary endpoints of IDFS and DDFS. The treatment lowered the likelihood of invasive breast cancer recurrence, secondary cancers, or death by 35% (HR 0.65; 95% CI 0.53-0.78), as well as the risk of distant disease recurrence or death by 35% (HR 0.65; 95% CI 0.53-0.81) compared to placebo. The advantages of LYNPARZA were seen consistently across all major subgroups, including those with high-risk, hormone receptor-positive conditions.
Judy E. Garber, who heads the Division of Cancer Genetics and Prevention at Dana-Farber Cancer Institute and serves as a co-principal investigator for the trial, stated: "These promising long-term findings from OlympiA validate that one year of adjuvant treatment with olaparib continues to provide significant survival benefits for patients with germline BRCA-mutated high-risk HER2-negative early breast cancer, even after six years. This benefit is persistent across all subgroups, and the toxicity and pregnancy data is reassuring for this generally younger population. These results highlight the necessity of germline BRCA testing at diagnosis to ensure that all qualifying patients can receive olaparib treatment as soon as possible."
Susan Galbraith, Executive Vice President of Oncology R&D at AstraZeneca, commented: "Two years ago, LYNPARZA was the first and only PARP inhibitor to show a survival advantage in patients with germline BRCA-mutated, HER2-negative, high-risk early-stage breast cancer. The continued demonstration of this benefit at the six-year mark is remarkable and emphasizes how LYNPARZA is playing a pivotal role in the evolution of treatment for early-stage breast cancer with BRCA mutations."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
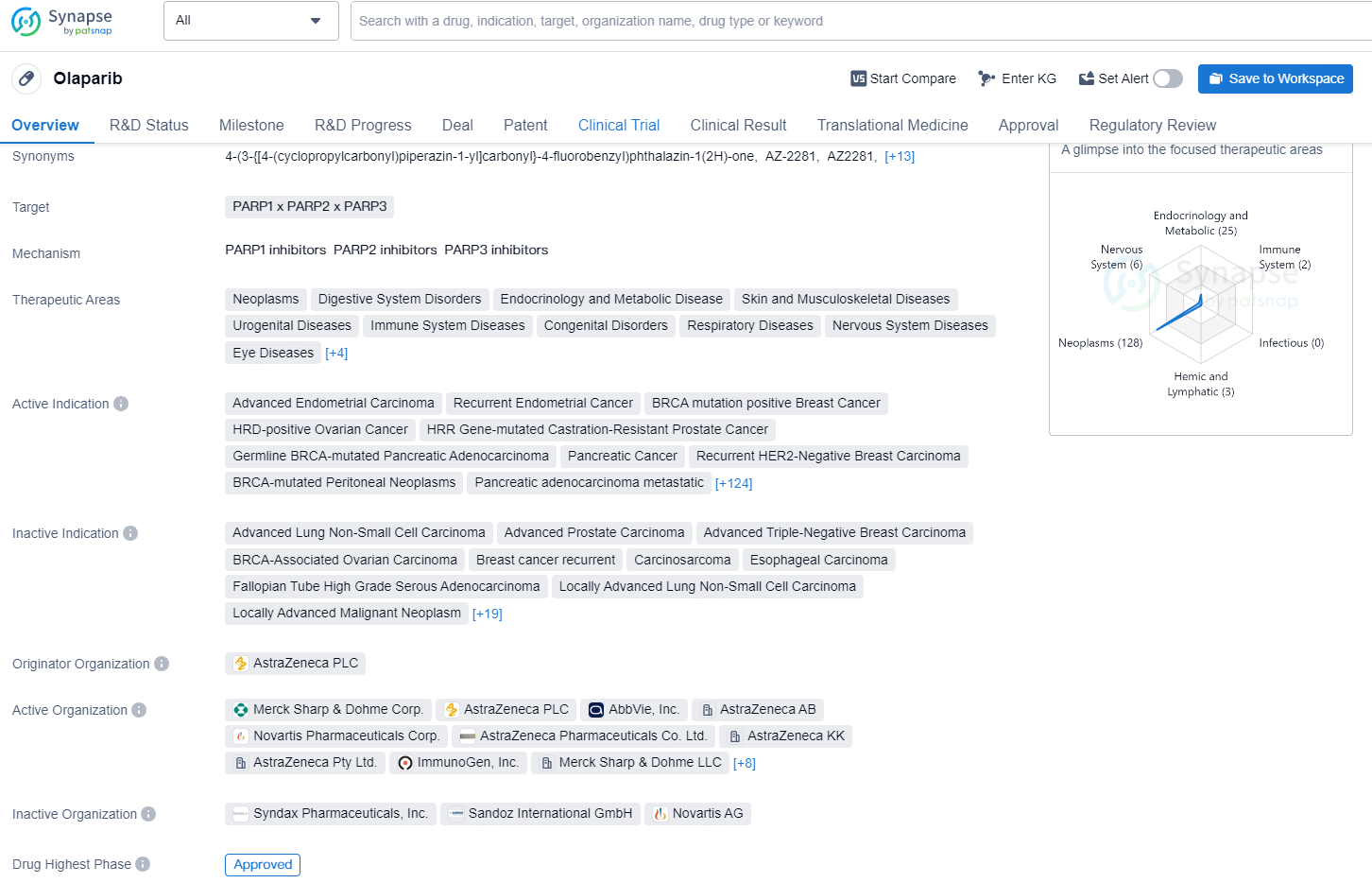
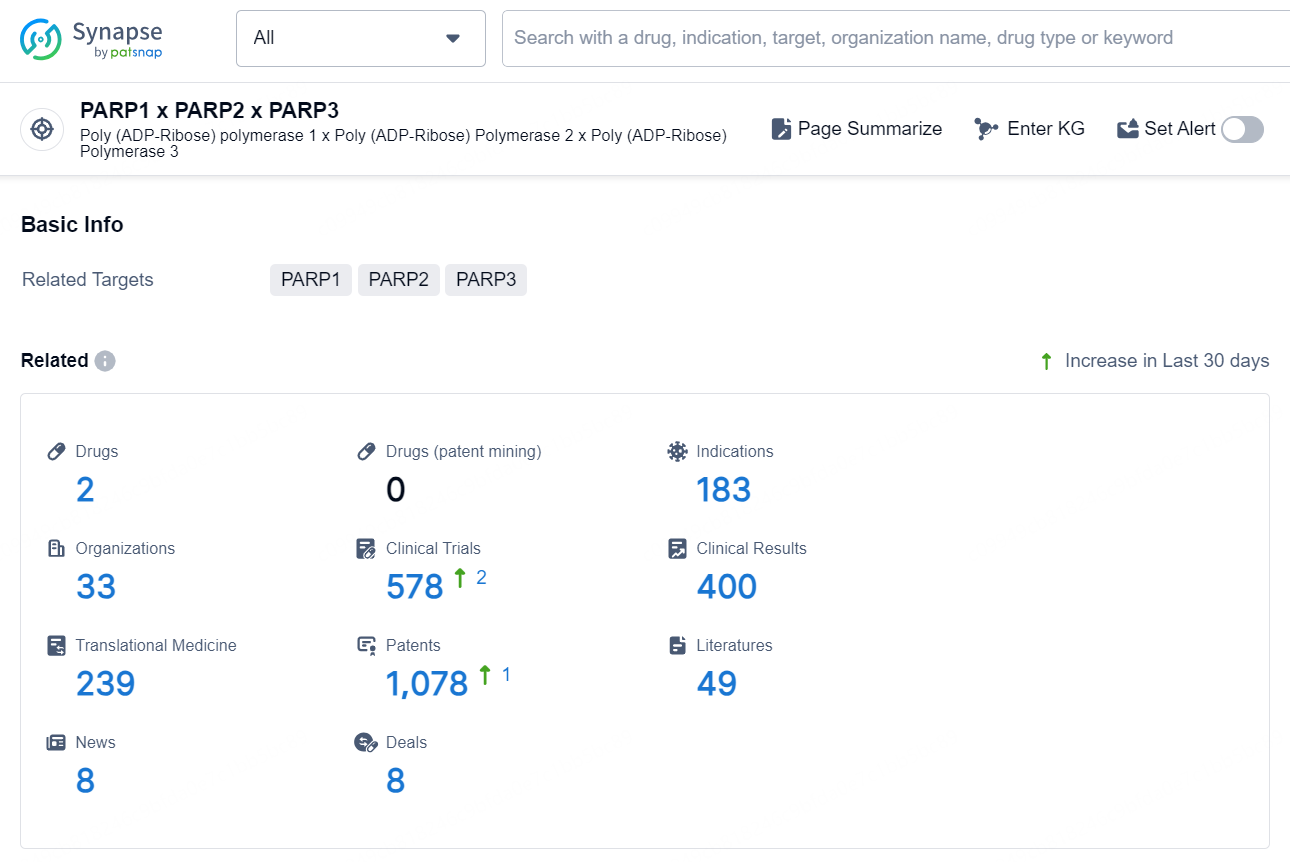
According to the data provided by the Synapse Database, As of December 18, 2024, there are 2 investigational drug for the PARP1 x PARP2 x PARP3 targets, including 183 indications, 33 R&D institutions involved, with related clinical trials reaching 578, and as many as 1078 patents.
Olaparib is a small molecule drug that targets PARP1, PARP2, and PARP3 and is used in the treatment of a wide range of therapeutic areas, including neoplasms, digestive system disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, urogenital diseases, immune system diseases, congenital disorders, respiratory diseases, nervous system diseases, and various other diseases affecting the eyes, mouth, teeth, and ears.