Caliway's CBL-514 Phase 2b Trial Shows Success in Reducing Abdominal Fat
Caliway Biopharmaceuticals (Caliway) has released the findings from the Phase 2b study of CBL-514 focused on reducing subcutaneous fat (CBL-0204 Phase 2b study, NCT05736107), demonstrating success in all primary and secondary efficacy endpoints within the Full Analysis Set (FAS) and Per-Protocol (PP) populations. Importantly, the primary endpoint results for the FAS population indicated that 76.7% of participants who received CBL-514 achieved at least a 1-grade improvement, as evaluated by the Investigator through the Clinician Reported-Abdominal Fat Rating Scale (CR-AFRS), compared to only 18.9% in the placebo group (p=0.00004). Furthermore, the study indicated a positive safety and tolerability profile, setting the stage for a global multi-center pivotal Phase 3 trial projected for 2025, with the potential of becoming the first injectable approved for significant fat reduction in larger areas.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
BL-0204 is a multi-center, randomized, placebo-controlled Phase 2b trial designed to assess the efficacy and safety of CBL-514 in the reduction of abdominal subcutaneous fat. A total of 107 participants with moderate to severe subcutaneous fat in the abdominal region, as measured by the Abdominal Fat Rating Scale (AFRS), were included in the trial. Participants were randomly assigned in a 1:1 ratio to receive either CBL-514 or a placebo, completing up to four treatment courses administered once every three weeks. Follow-up evaluations were scheduled at 4, 8, and 12 weeks post-treatment, with a maximum dosage of 600 mg per session.
In order to increase the likelihood of achieving the efficacy objectives in the upcoming pivotal Phase 3 study of CBL-514, the CBL-0204 trial utilized the efficacy assessment tools recommended by the U.S. FDA, including AFRS and MRI (Magnetic Resonance Imaging), to evaluate changes in subcutaneous fat volume in the targeted area.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
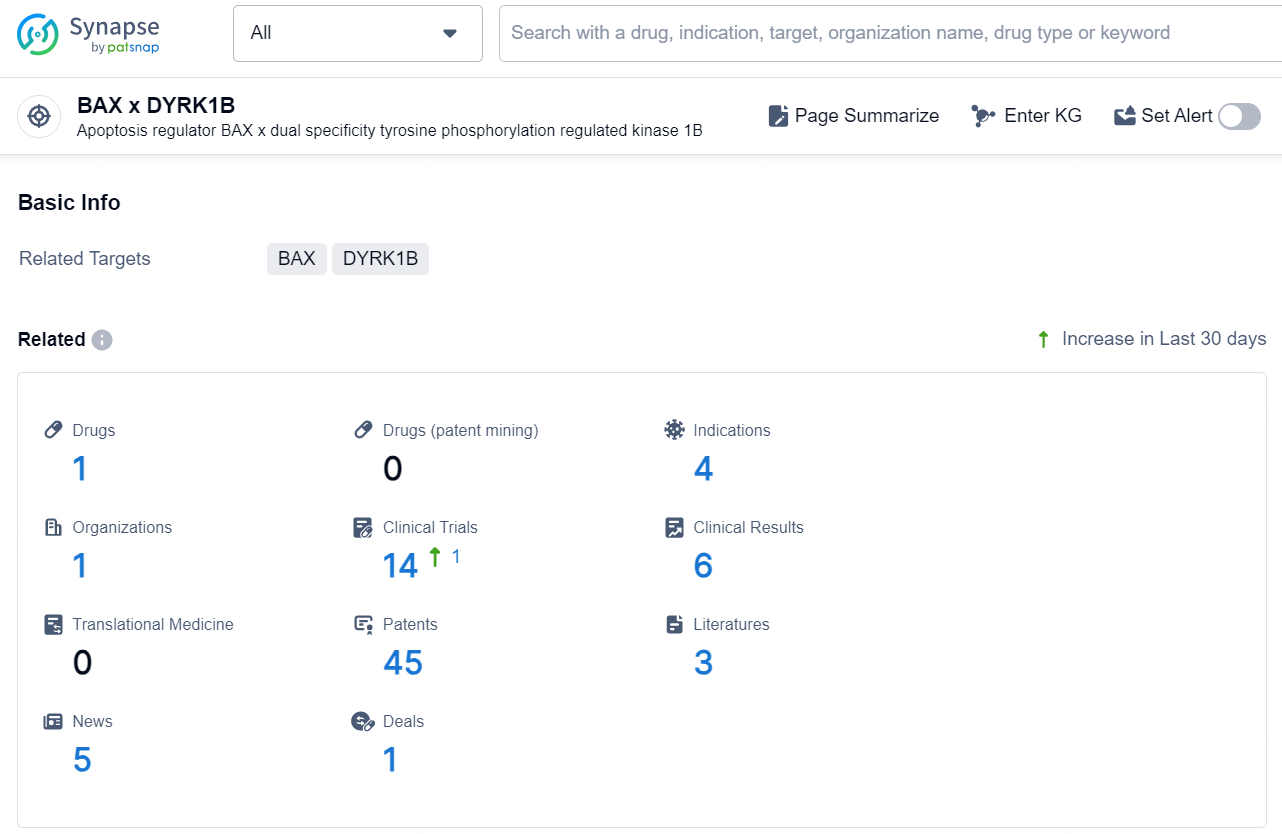
According to the data provided by the Synapse Database, As of December 18, 2024, there are 1 investigational drug for the BAX x DYRK1B target, including 4 indications, 1 R&D institution involved, with related clinical trials reaching 14, and as many as 45 patents.
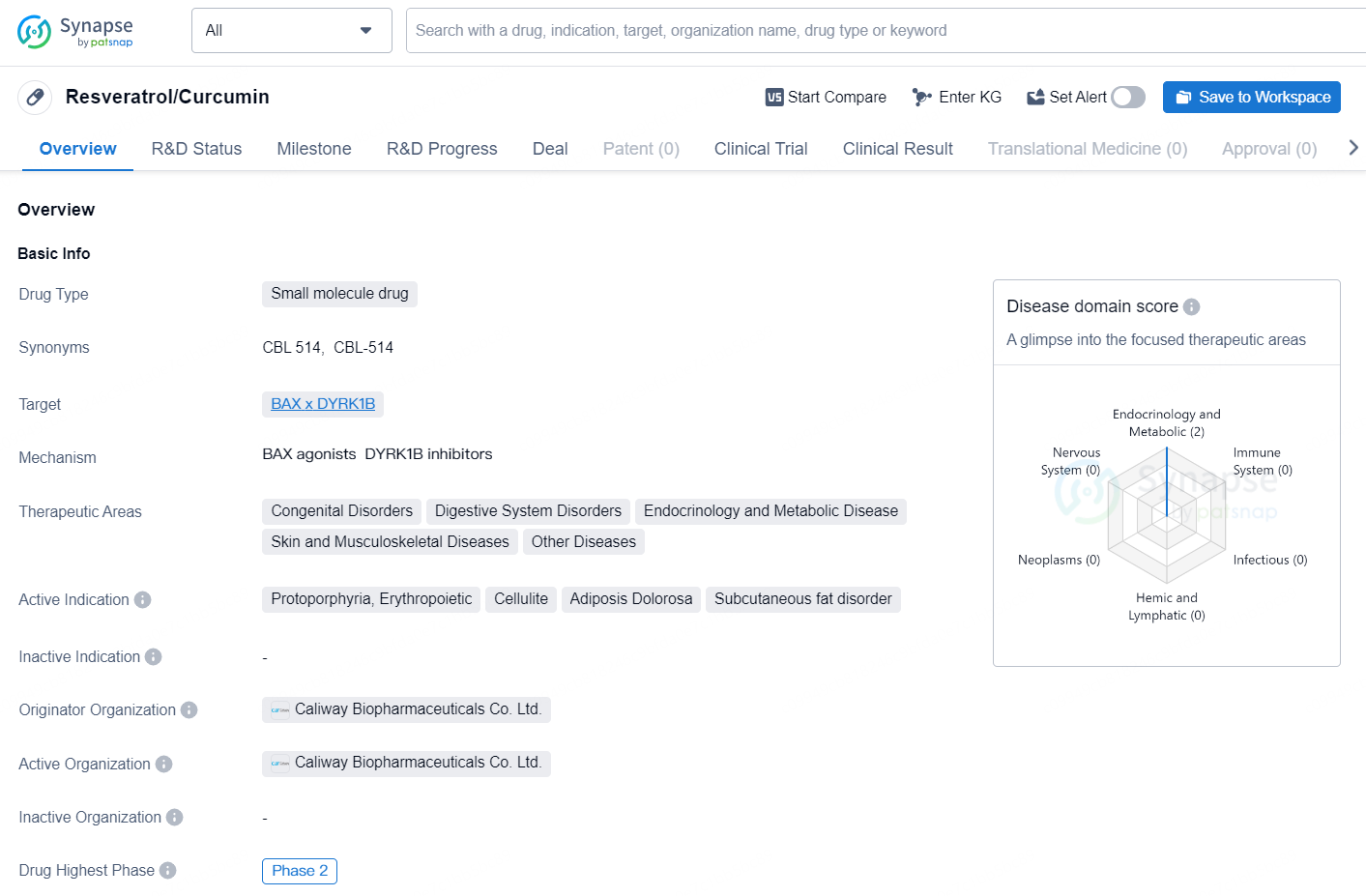
Resveratrol/Curcumin is a small molecule drug that targets BAX x DYRK1B. It is being developed for the treatment of a range of therapeutic areas including congenital disorders, digestive system disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, as well as other diseases. The active indications for Resveratrol/Curcumin include protoporphyria, erythropoietic, cellulite, adiposis dolorosa, and subcutaneous fat disorder. The drug is being developed by Caliway Biopharmaceuticals Co. Ltd., and it is currently in Phase 2 of clinical development.