Merck's KEYLYNK-001 Study Achieves Main Goal in Advanced Ovarian Cancer
Merck (NYSE: MRK), referred to as MSD in regions outside the United States and Canada, has declared that the Phase 3 KEYLYNK-001 study, which assessed the combination of KEYTRUDA® (pembrolizumab) and chemotherapy followed by LYNPARZA® (olaparib) maintenance, with or without bevacizumab, as a primary treatment for individuals with BRCA non-mutated advanced epithelial ovarian cancer, has achieved its main goal of progression-free survival (PFS). The final analysis, performed by an independent Data Monitoring Committee, revealed that the KEYTRUDA and LYNPARZA treatment plan significantly enhanced PFS for these patients compared to chemotherapy alone, marking a statistically and clinically significant improvement.
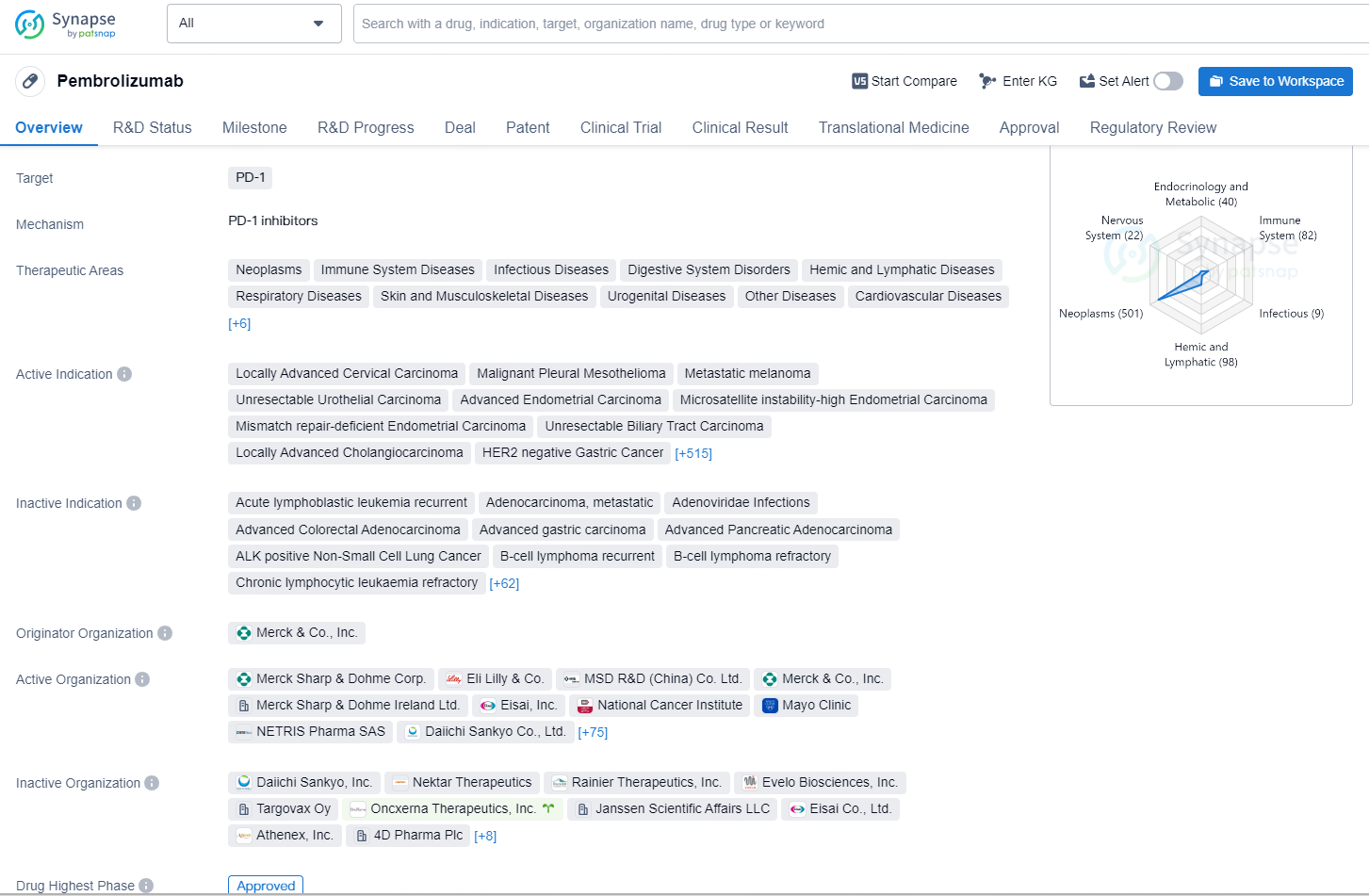
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The trial failed to achieve its secondary endpoint of overall survival (OS). The effectiveness of KEYTRUDA in the intention-to-treat group is still unclear. The safety profiles for KEYTRUDA and LYNPARZA align with those from previous studies on the individual treatments. These findings will be showcased at a forthcoming medical conference and reviewed with regulatory bodies.
"Individuals with ovarian cancer continue to face a significant need for novel treatments that could enhance outcomes," stated Dr. Gursel Aktan, Vice President of Global Clinical Development at Merck Research Laboratories. "KEYLYNK-001 marks the first successful Phase 3 trial combining KEYTRUDA and LYNPARZA, underscoring our dedication to research aimed at mitigating the global burden of women's cancers."
In the United States, LYNPARZA is approved for three indications in ovarian cancer: for maintaining treatment in adult patients with harmful or suspected harmful germline or somatic BRCA-mutated (gBRCAm or sBRCAm) advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who have responded completely or partially to first-line platinum-based chemotherapy; in conjunction with bevacizumab for maintenance in adult patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who have responded to first-line platinum-based chemotherapy and whose cancer is HRD-positive, as indicated by a harmful or suspected harmful BRCA mutation, and/or genomic instability; and for maintenance in adult patients with harmful or suspected harmful gBRCAm or sBRCAm recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who have responded to platinum-based chemotherapy. For each indication, therapy selection is based on an FDA-approved companion diagnostic for LYNPARZA.
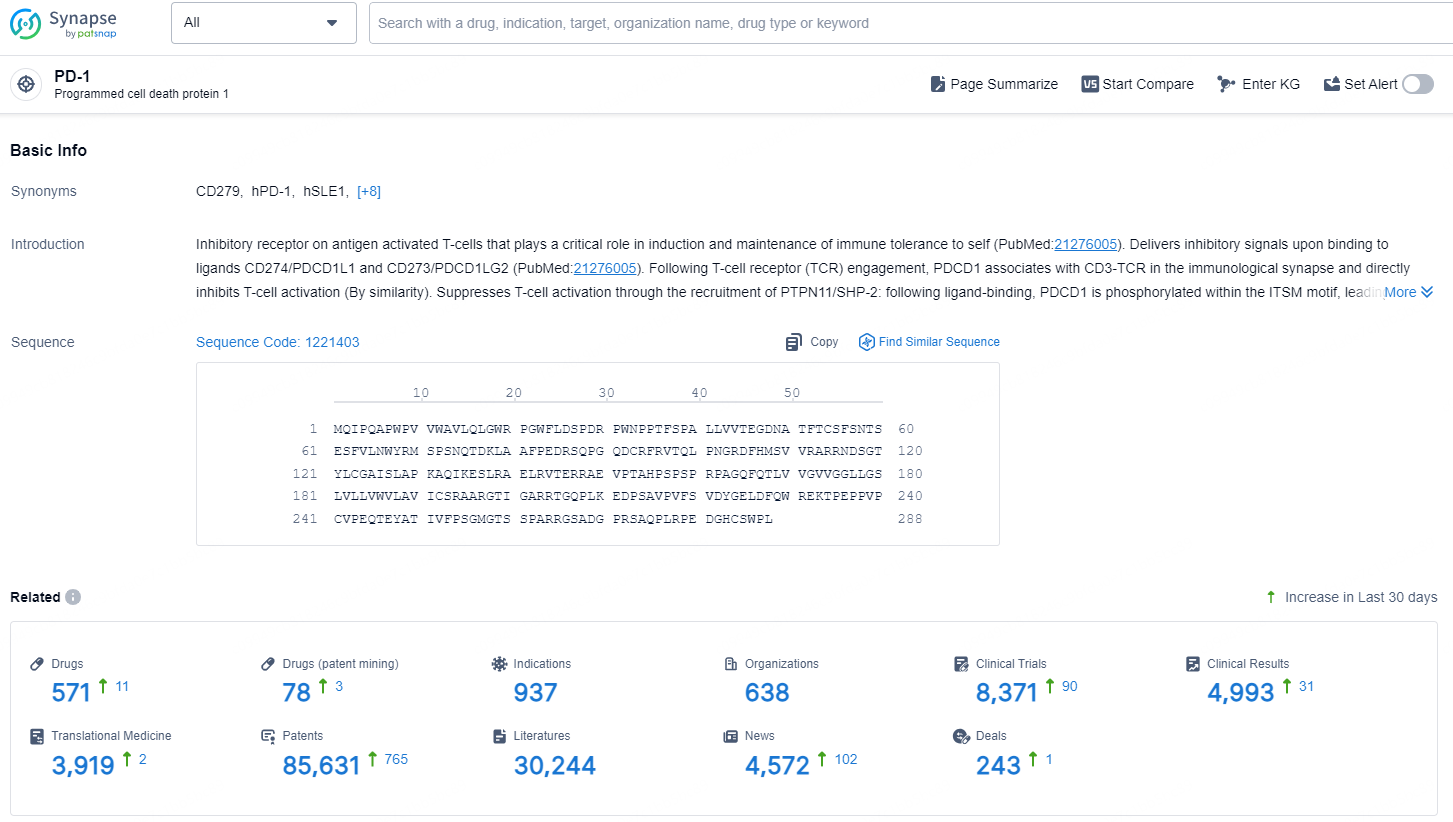
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 12, 2024, there are 571 investigational drug for the PD-1 targets, including 937 indications, 638 R&D institutions involved, with related clinical trials reaching 8371, and as many as 85631 patents.
The drug Pembrolizumab is a monoclonal antibody that targets the PD-1 protein and is approved for the treatment of a wide range of therapeutic areas including neoplasms, immune system diseases, infectious diseases, digestive system disorders, and many others. It is indicated for the treatment of various types of cancer such as locally advanced cervical carcinoma, metastatic melanoma, unresectable urothelial carcinoma, and advanced endometrial carcinoma, among others.