Molecular Partners Shares MP0533 Clinical Updates and MP0621 Preclinical Insights at ASH 2024
Molecular Partners AG, a clinical-stage biotechnology firm specializing in the development of DARPin therapeutics—a novel class of engineered protein drugs-has disclosed the presentation of further data from two of its programs. This includes preclinical findings on MP0621, which is being explored as a next-generation conditioning regimen for patients receiving hematopoietic stem cell transplants, and detailed results from the initial seven cohorts of the ongoing phase 1/2a dose-escalation study of MP0533. These data are featured in two posters at the annual meeting of the American Society of Hematology (ASH), taking place from December 7 to 10, 2024, in San Diego, CA.
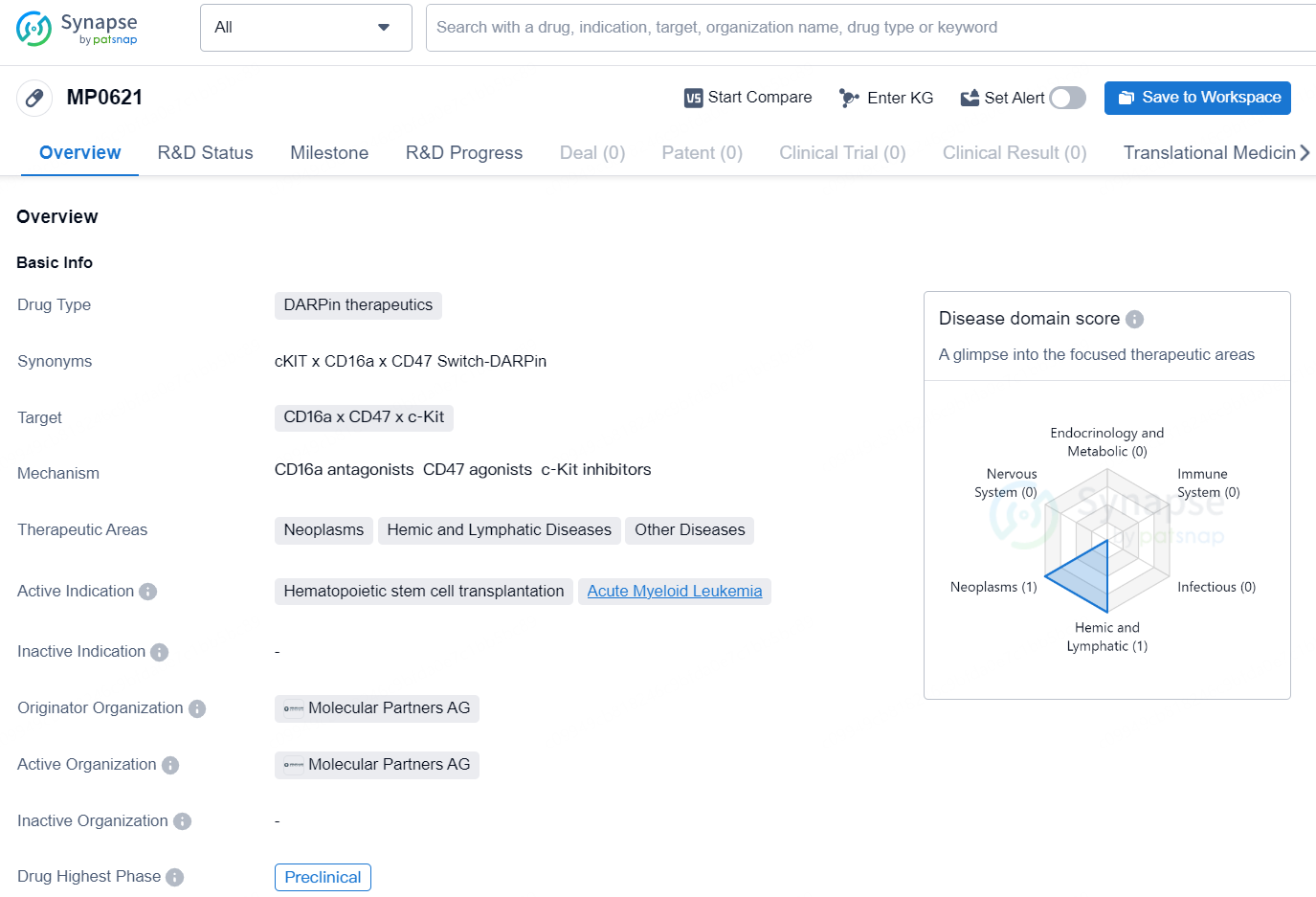
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
MP0621 is a Switch-DARPin candidate engineered to target and eliminate hematopoietic stem cells (HSCs) as part of a next-generation conditioning strategy for HSC transplantation (HSCT). The Switch-DARPin technology incorporates a logic-gated "on/off" mechanism, enabling target activation only in the presence of specific antigens. In MP0621, the Switch-DARPin can bind to cellular cKit or the anti-CD47 DARPin binder. When MP0621 attaches to cKit on HSCs, it reveals the anti-CD47 DARPin, which then binds to CD47 and inhibits the "don't-eat-me" signal, harnessing the benefits of CD47 inhibition without toxicity to healthy cells.
The preclinical data presented confirm the intended mechanism of MP0621 and offer additional support for its potential as an effective method for HSC depletion in patients. By selectively blocking CD47 on target cells, MP0621 enhances the efficacy of cKit-targeting and minimizes off-target effects associated with systemic anti-CD47 blockade. However, the current non-human primate data do not enable Molecular Partners to confirm MP0621's effectiveness as a treatment for AML patients, as was previously hypothesized. Given Molecular Partners' focus on oncology therapeutic candidates, MP0621 is under evaluation for potential partnerships.
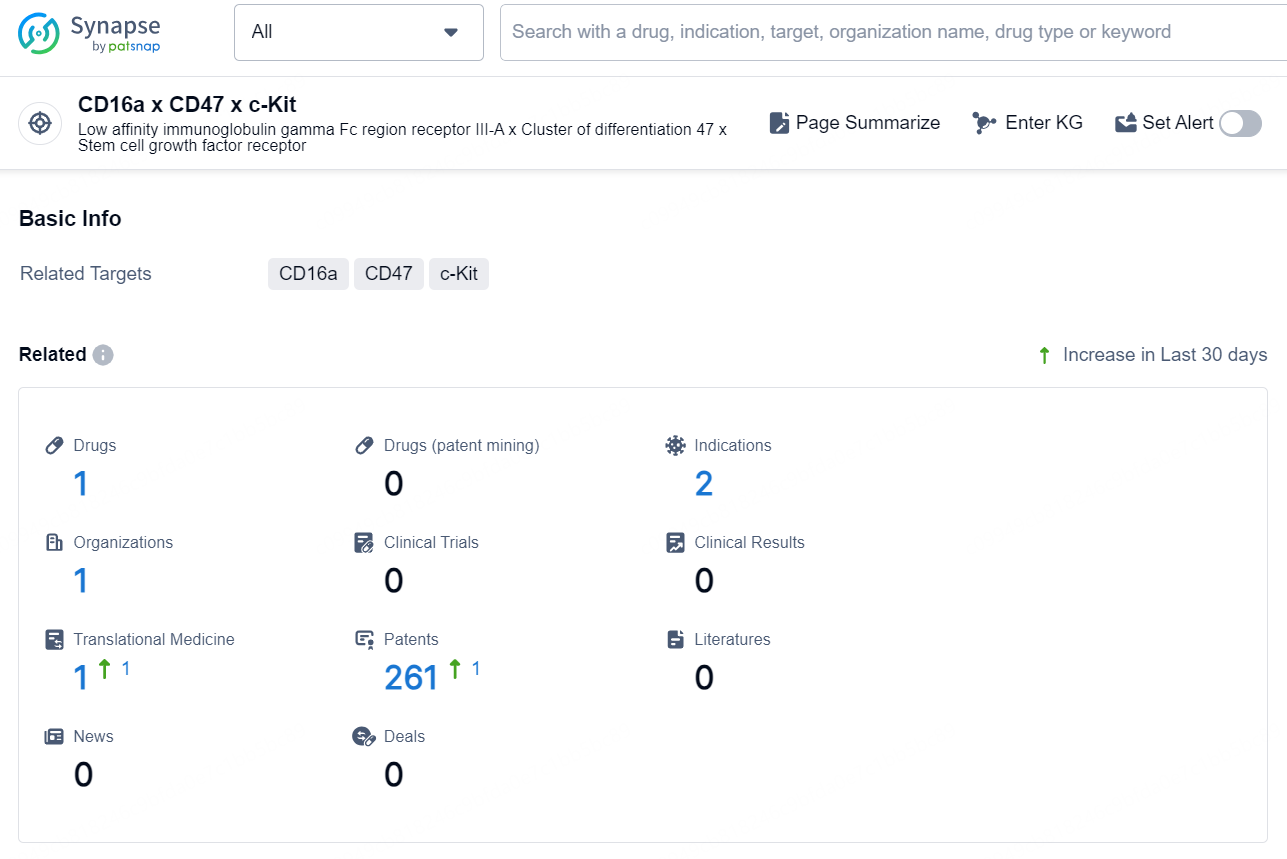
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 12, 2024, there are 1 investigational drug for the CD16a x CD47 x c-Kit target, including 2 indications, 1 R&D institution involved, and as many as 261 patents.
MP0621 is a DARPin therapeutic drug developed by Molecular Partners AG, with its highest phase of development being Preclinical. This drug targets CD16a, CD47, and c-Kit, making it a potential candidate for therapeutic areas such as Neoplasms, Hemic and Lymphatic Diseases, and Other Diseases. The active indications for this drug are Hematopoietic stem cell transplantation and Acute Myeloid Leukemia.