MTTI 225Ac-EBTATE Proves Extremely Potent in Combating Neuroendocrine Tumors
Molecular Targeting Technologies, Inc. (MTTI) has released preclinical research findings for their proprietary 225Ac-EBTATE targeting SSTR2-positive neuroendocrine tumors (NET) in the European Journal of Nuclear Medicine. The study, titled "Long-acting 225Ac-EBTATE demonstrates high efficacy against somatostatin receptor-2-positive neuroendocrine tumors," is available online (https://rdcu.be/d2lCG).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
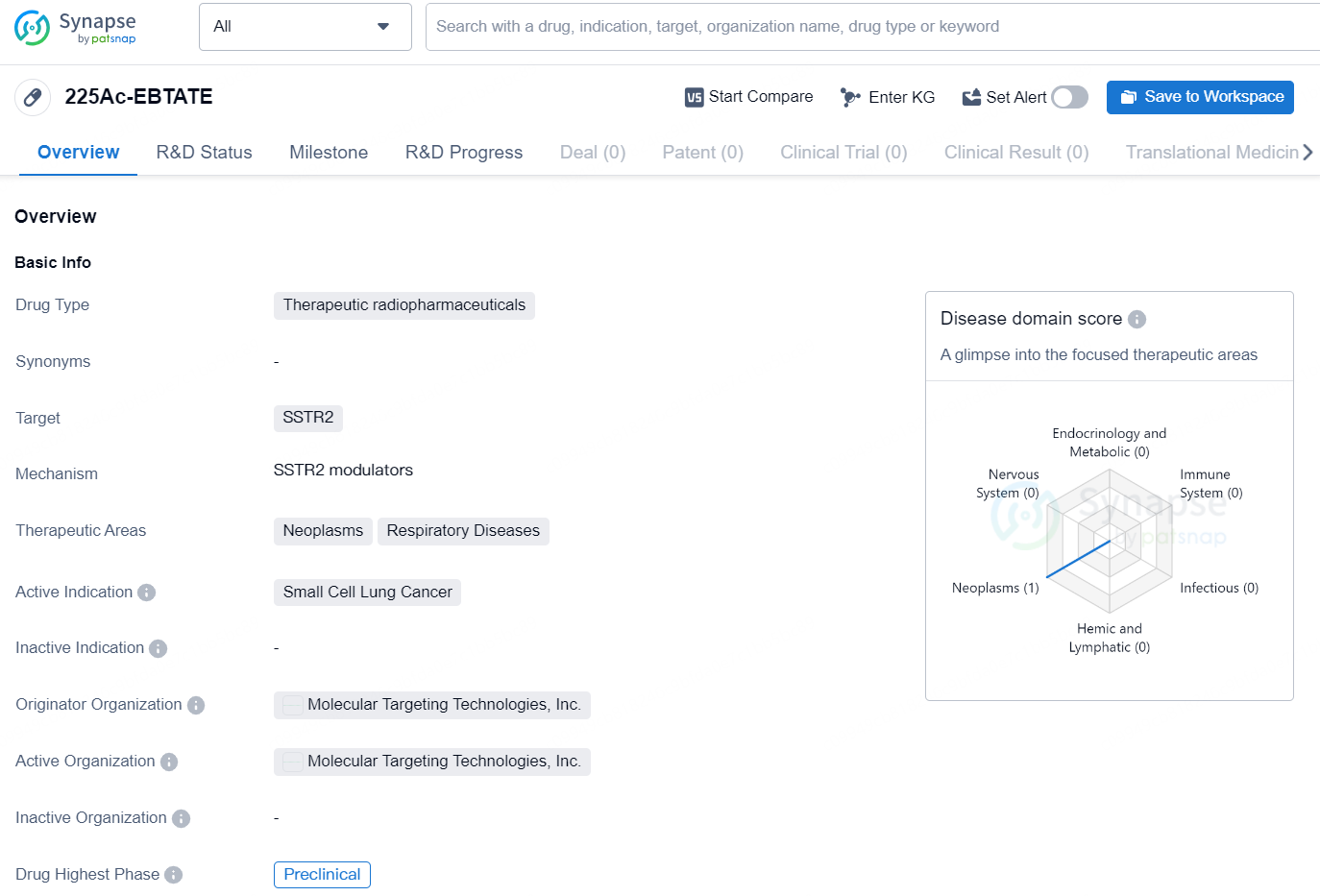
Professor Humphrey Fonge from Université de Laval and the lead author stated, "With only 40% of the dose used for 225Ac-DOTATATE, 225Ac-EBTATE has shown superior anti-tumor effects in Small Cell lung cancer (SCLC) and Pan-neuroendocrine tumor (NET) models, achieving complete tumor remissions. At a therapeutic dose of 2x 34 kBq, 225Ac-EBTATE exhibited general safety over 28 days based on blood biochemistry analysis, CBC, and histopathological examination of major organs and tissues. Our results indicate that 225Ac-EBTATE is effective against human small-cell lung cancer (SCLC), with an 80% complete remission rate and 100% survival in preclinical models."
MTTI has secured commercial use rights for the patented Evans blue (EB) technology from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), a division of the National Institutes of Health (NIH). This technology forms the foundation for a best-in-class, new generation of targeted radiopharmaceuticals (TRP). EB's binding to serum albumin prolongs in vivo circulatory half-life and tumor residence time, enhancing tumor uptake by up to 30-fold and allowing for effective treatment with significantly lower radiopharmaceutical activity and fewer dosing cycles compared to the current standard of care. Our 3-year follow-up of 177Lu-EBTATE in patients* (N=30) with metastatic neuroendocrine tumors showed good safety with no nephro- or hepatoxicity and an 86% disease control rate using only 40% of the administered dose of 177Lu-DOTATATE.
Dr. Jean-Mathieu Beauregard, MD, Associate Professor in the Department of Radiology at Université Laval, expressed, "I am optimistic about the positive outcomes of 225Ac-EBTATE in preclinical studies for treating small cell lung cancer and Pan-neuroendocrine tumor (NET). I am eager to collaborate with MTTI and Professor Fonge to transition these findings into clinical practice."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
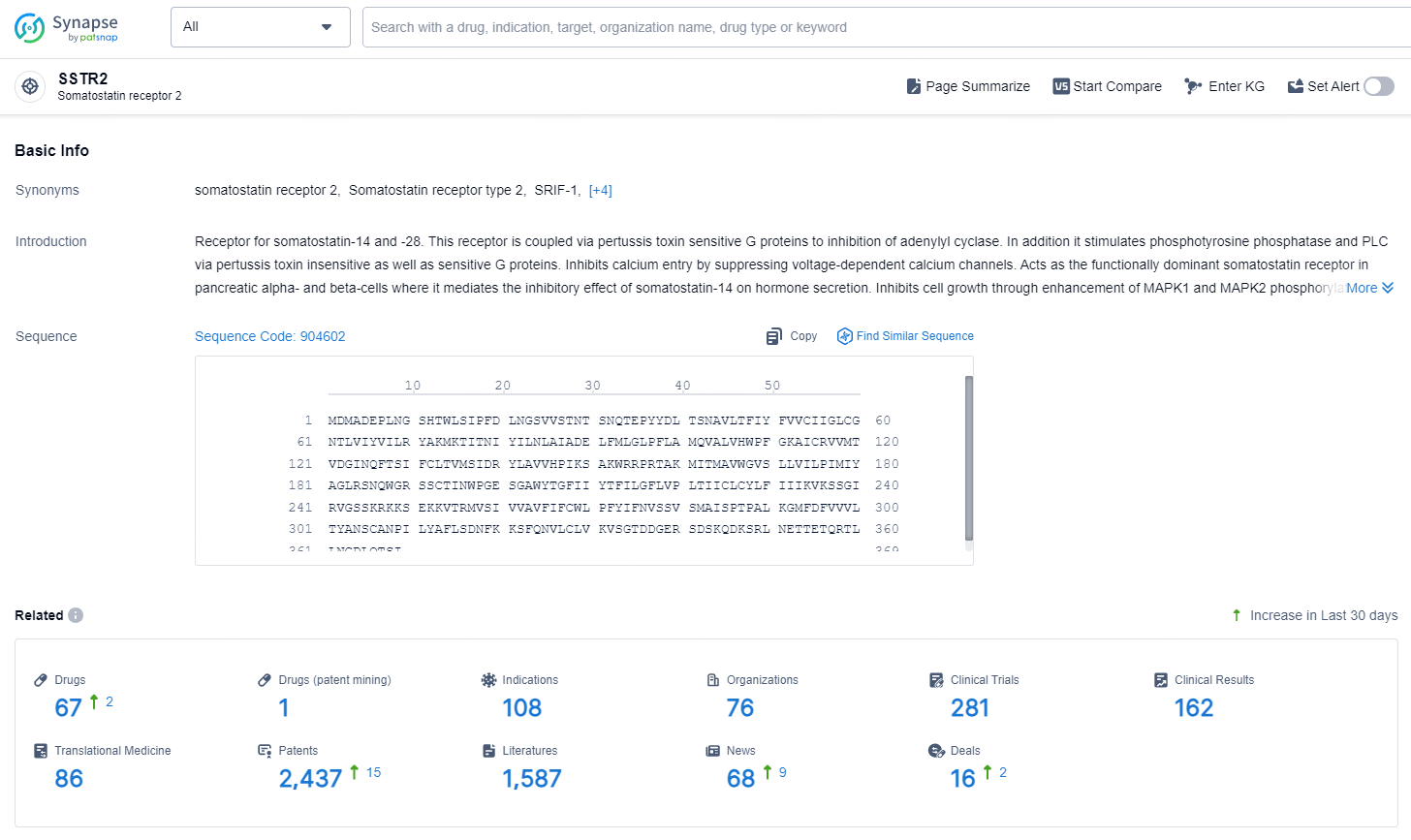
According to the data provided by the Synapse Chemical, As of December 12, 2024, there are 67 investigational drugs for the SSTR2 target, including 108 indications, 78 R&D institutions involved, with related clinical trials reaching 281, and as many as 2437 patents.
225Ac-EBTATE is a therapeutic radiopharmaceutical drug designed to target SSTR2, primarily focused on treating neoplasms and respiratory diseases, with a specific indication for small cell lung cancer. The drug is currently in the preclinical stage and is developed by Molecular Targeting Technologies, Inc.