U.S. Approves TRYNGOLZA™ (olezarsen) as First Treatment for Familial Chylomicronemia Syndrome in Adults
Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) has announced that the U.S. Food and Drug Administration (FDA) has granted approval for TRYNGOLZA™ (olezarsen) as a supplementary treatment along with diet to lower triglyceride levels in adults suffering from familial chylomicronemia syndrome (FCS), a rare genetic condition characterized by severe hypertriglyceridemia (sHTG) that can potentially result in life-threatening acute pancreatitis (AP). This marks the first FDA-approved therapy that significantly and substantially decreases triglyceride levels in adults with FCS, while also offering a clinically significant reduction in AP incidents when administered in conjunction with an appropriate diet (limited to ≤20 grams of fat daily). TRYNGOLZA is designed for self-administration using an auto-injector on a monthly basis.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The FDA's approval of TRYNGOLZA today marks a significant milestone as it introduces the first FCS treatment available in the U.S., representing a transformative development for patients and their families. "For the first time, adults suffering from FCS can access a treatment that significantly lowers triglyceride levels and mitigates the risk of debilitating and potentially life-threatening acute pancreatitis," stated Brett P. Monia, Ph.D., CEO of Ionis. "We cherish our enduring collaboration with the FCS community and extend our gratitude to the patients, families, and researchers who contributed to our clinical trials, which made this treatment possible. Moreover, the FDA's endorsement of TRYNGOLZA signifies a critical step in Ionis’s journey towards becoming a fully integrated commercial biotechnology firm, a goal we set out to realize five years ago. With an extensive pipeline of transformative therapies, we anticipate that TRYNGOLZA will be the first in a series of groundbreaking medicines we will introduce independently for individuals facing serious health challenges."
The FDA's decision was supported by favorable outcomes from the global, multicenter, randomized, placebo-controlled, double-blind Phase 3 Balance clinical trial that involved adult patients with genetically confirmed FCS and fasting triglyceride levels at or above 880 mg/dL. In this study, TRYNGOLZA at a dose of 80 mg showed a statistically significant placebo-adjusted average triglyceride reduction of 42.5% from baseline after six months (p=0.0084). Over the course of 12 months, reductions from baseline improved further, with TRYNGOLZA achieving a placebo-adjusted mean reduction of 57% in triglyceride levels. Additionally, TRYNGOLZA led to a significant and clinically relevant decrease in acute pancreatitis (AP) occurrences over 12 months; only one patient (5%) in the TRYNGOLZA group had a single AP episode, while seven patients (30%) in the placebo group experienced a total of 11 AP episodes.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
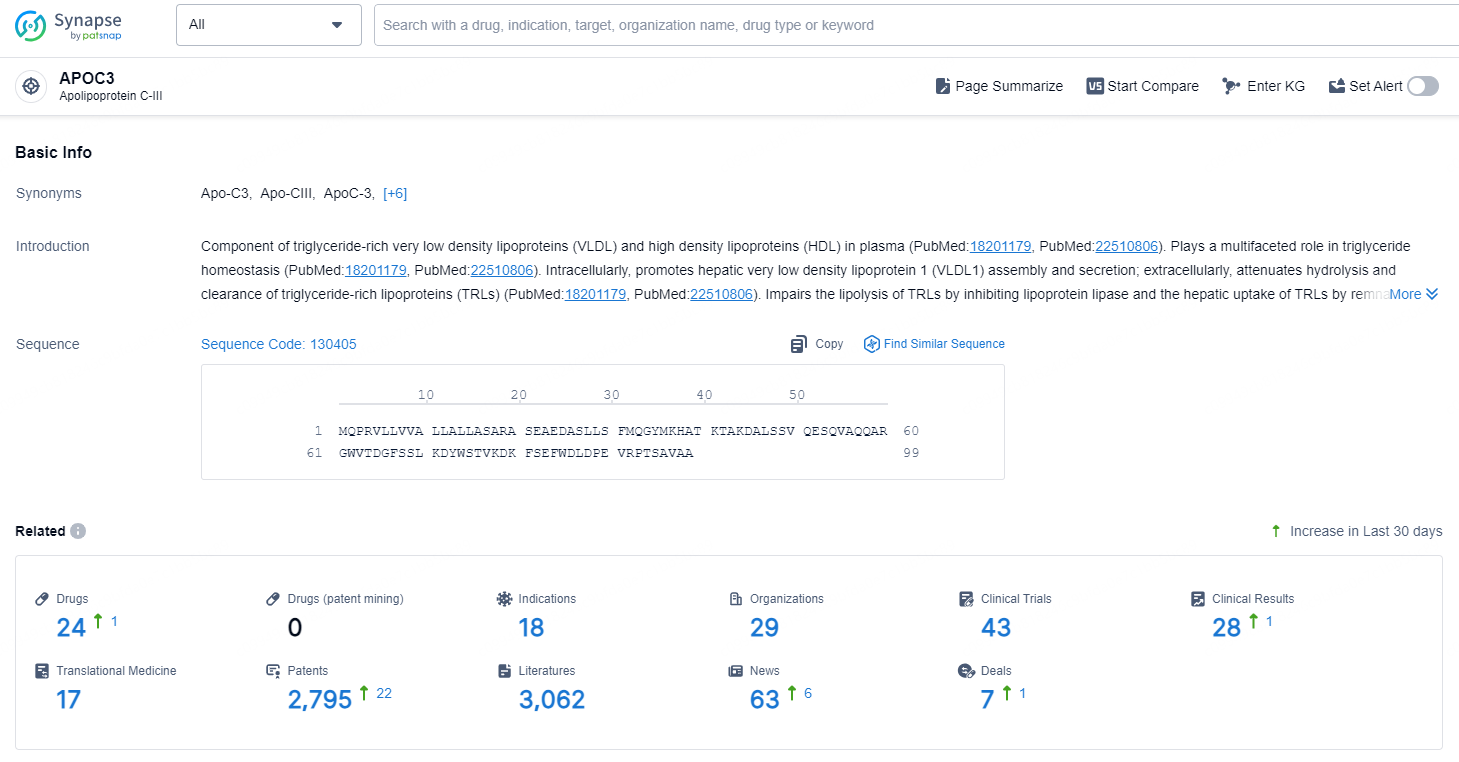
According to the data provided by the Synapse Chemical, As of December 24, 2024, there are 24 investigational drugs for the APOC3 target, including 18 indications, 29 R&D institutions involved, with related clinical trials reaching 43, and as many as 2795 patents.
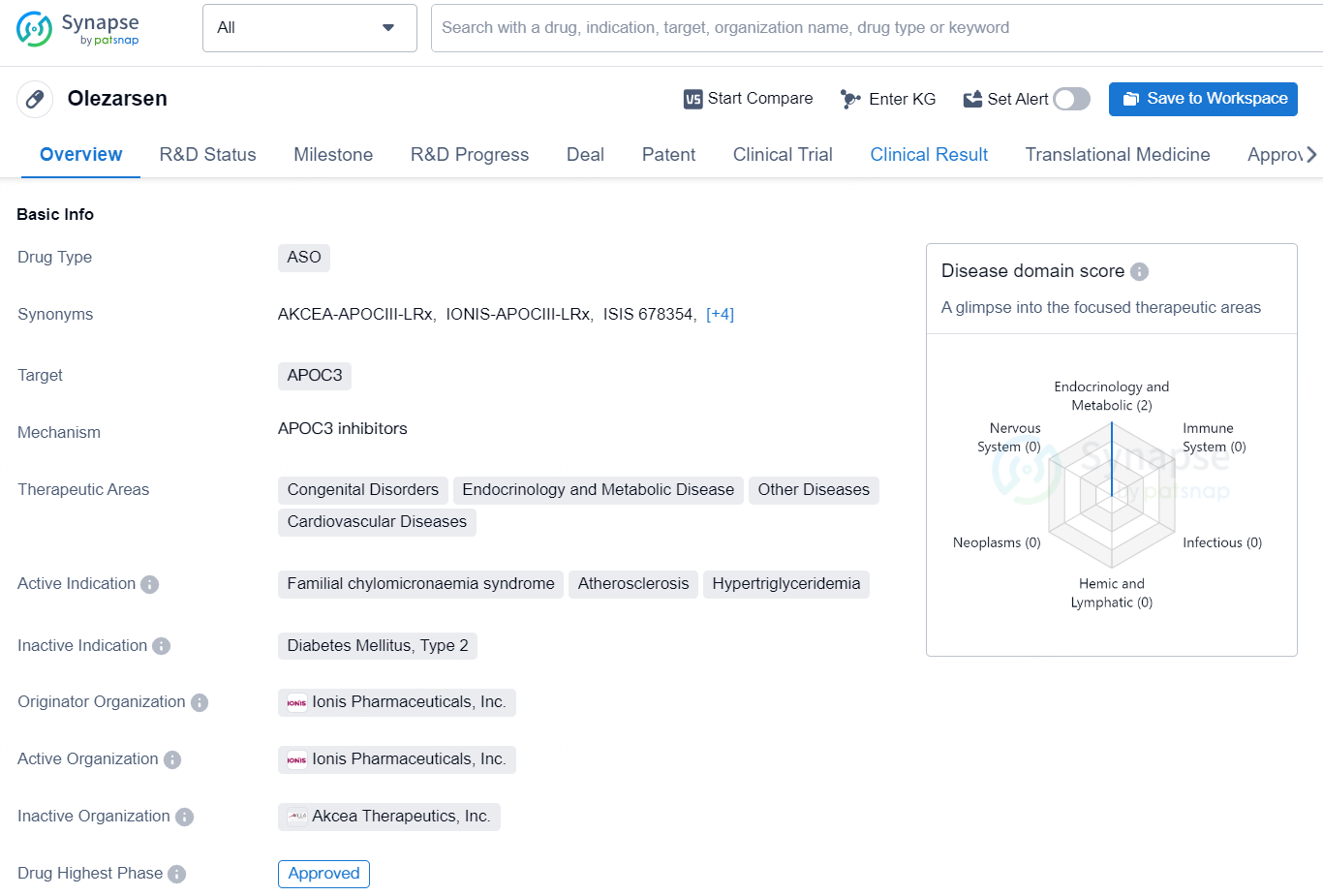
Olezarsen is an antisense oligonucleotide (ASO) drug developed by Ionis Pharmaceuticals, Inc. that targets the APOC3 gene. The therapeutic areas for which Olezarsen is indicated include Congenital Disorders, Endocrinology and Metabolic Disease, Other Diseases, and Cardiovascular Diseases. The drug is specifically indicated for the treatment of Familial chylomicronaemia syndrome, Atherosclerosis, and Hypertriglyceridemia.