Vertex Announces Phase 2 Results for Suzetrigine in Treating Painful Lumbar Radiculopathy
Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) reported findings from its Phase 2 clinical trial of suzetrigine, an experimental oral medication that selectively inhibits NaV1.8 pain signals for individuals suffering from painful lumbosacral radiculopathy (LSR). The trial achieved its primary objective, demonstrating a statistically significant and clinically relevant decrease in pain as measured by the numeric pain rating scale (NPRS).
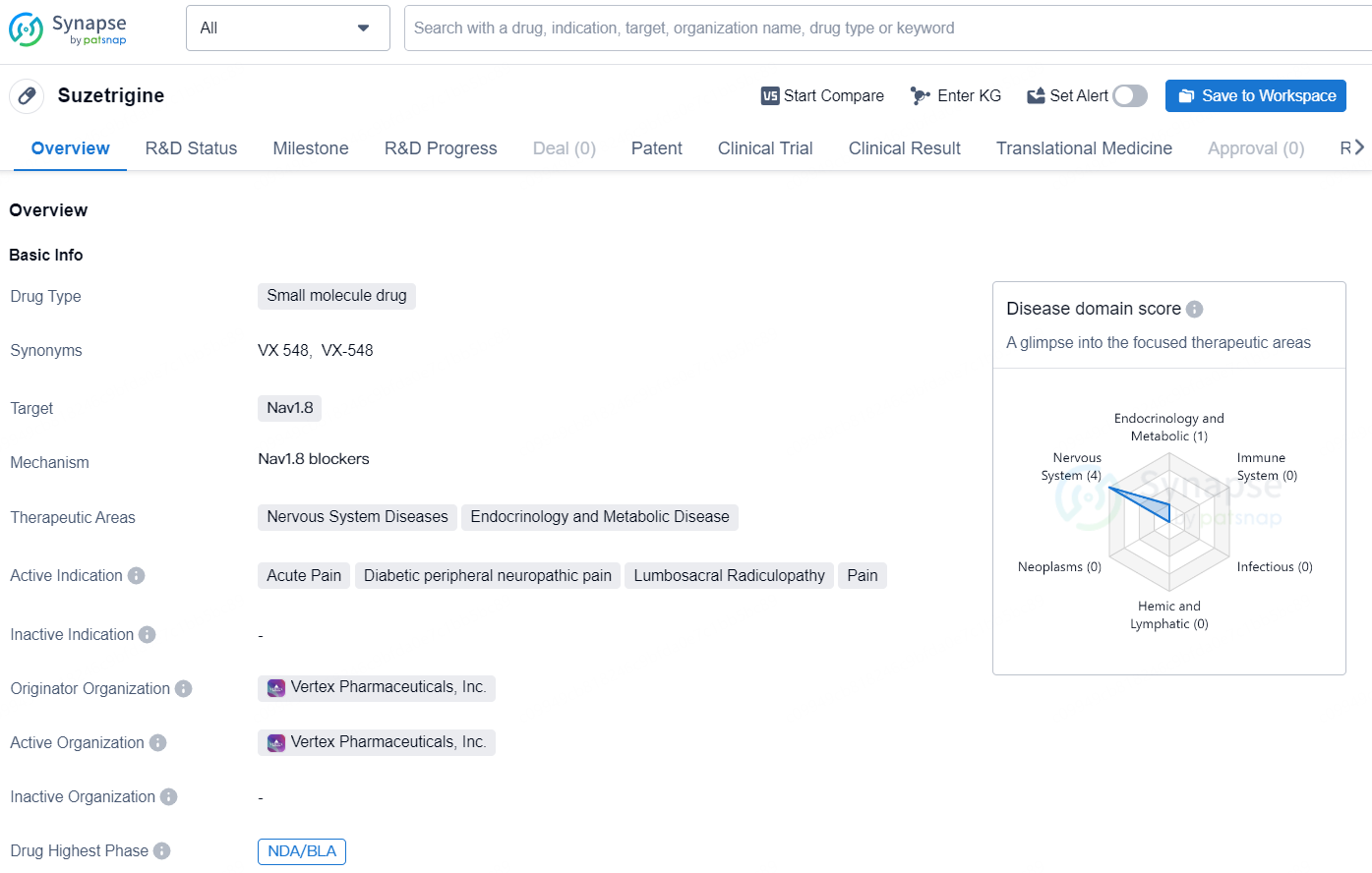
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The main objective of the study was to assess the change in the weekly average of leg pain intensity from baseline using the NPRS at Week 12. This scale spans from 0 (indicating no pain) to 10 (representing the most severe pain possible).
In the suzetrigine group, a significant and clinically relevant reduction in pain was observed, with a mean change in NPRS of -2.02 by Week 12. There was also a placebo group included in the study that demonstrated a similar reduction, with a mean change in NPRS of -1.98. However, the study was not structured nor had sufficient power to statistically compare the outcomes between the suzetrigine and placebo groups.
Overall, suzetrigine was found to be well tolerated throughout the study. The occurrence of adverse events (AEs) was recorded at 22.9% in the suzetrigine group compared to 32.4% in the placebo group. Most AEs reported in both groups were classified as mild to moderate in severity. Notably, there were no serious adverse events (SAEs) that were related or potentially related to suzetrigine, and no AEs resulted in treatment discontinuation among the participants receiving suzetrigine.
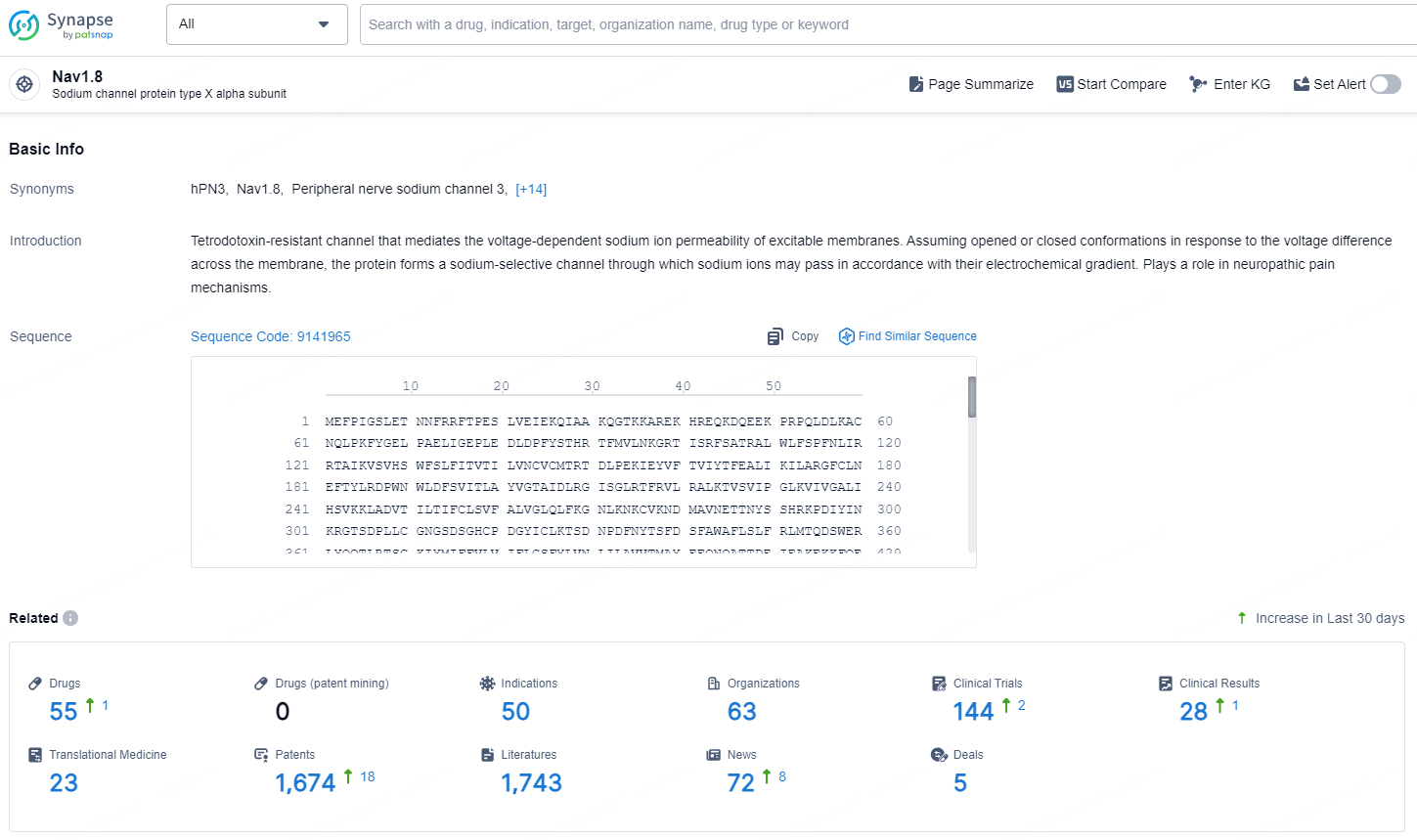
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 24, 2024, there are 55 investigational drug for the Nav1.8 targets, including 50 indications, 63 R&D institutions involved, with related clinical trials reaching 144, and as many as 1674 patents.
Suzetrigine is a small molecule drug developed by Vertex Pharmaceuticals, Inc. that targets Nav1.8, a sodium channel involved in the transmission of pain signals. The drug falls under the therapeutic areas of Nervous System Diseases and Endocrinology and Metabolic Disease, with active indications including Acute Pain, Diabetic peripheral neuropathic pain, Lumbosacral Radiculopathy, and Pain.