Teva and Alvotech Announce the U.S. Release of SIMLANDI® (adalimumab-ryvk) Injection
Teva Pharmaceuticals USA, a subsidiary of Teva Pharmaceutical Industries Ltd., together with Alvotech, have declared the launch of SIMLANDI (adalimumab-ryvk) injection within the United States. This product is an interchangeable biosimilar to Humira, indicated for treating a range of conditions including rheumatoid arthritis in adults, juvenile idiopathic arthritis, psoriatic arthritis in adults, ankylosing spondylitis in adults, Crohn's disease, ulcerative colitis in adults, plaque psoriasis in adults, hidradenitis suppurativa in adults, and uveitis in adults.
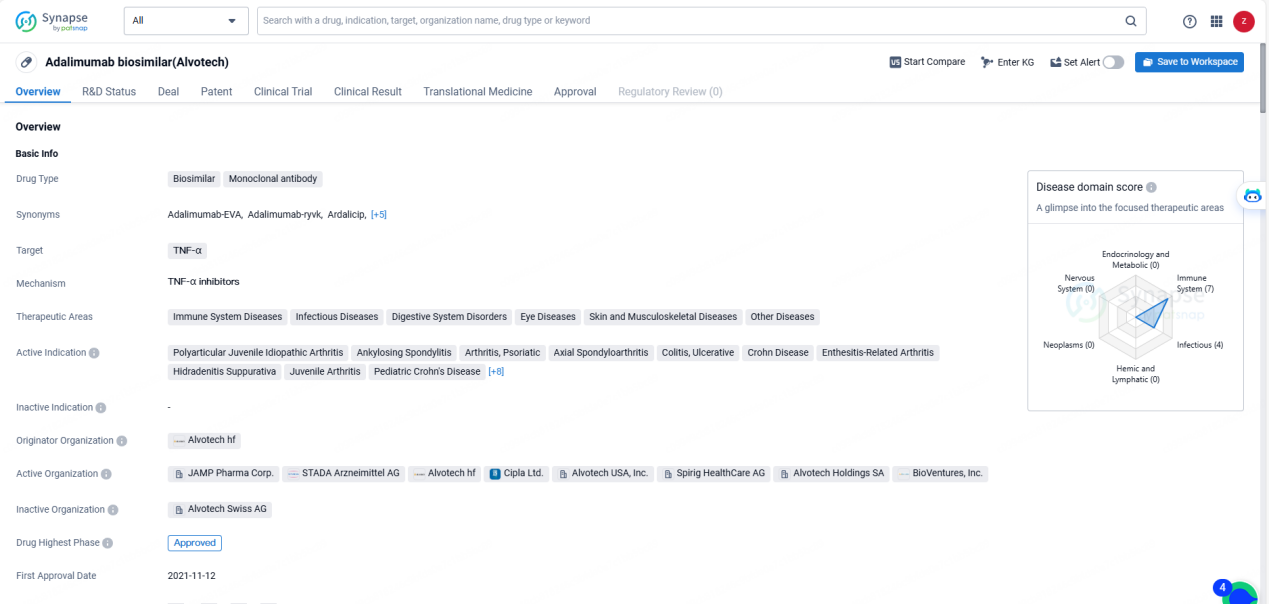
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We are excited to introduce SIMLANDI to patients and healthcare providers in the U.S.," stated Thomas Rainey, Senior Vice President, U.S. Market Access at Teva. "Biosimilars offer significant cost-saving opportunities within the healthcare system, and the availability of SIMLANDI—a high-concentration, citrate-free biosimilar now designated as interchangeable with Humira—marks an important milestone in the U.S. We will collaborate with payors to ensure SIMLANDI's accessibility, alongside six other biosimilars we aim to bring to market by 2027."
The U.S. Food and Drug Administration has approved SIMLANDI as the inaugural high-concentration, citrate-free biosimilar to Humira, with exclusivity for interchangeability for the 40mg/0.4mL injection. Currently, both low-concentration and high-concentration biosimilars of Humira are available in the U.S., with approximately 88 percent of adalimumab prescriptions being for the high-concentration format.
"It's a tremendous honor to offer U.S. patients SIMLANDI, the sole citrate-free, high-concentration interchangeable biosimilar to Humira," remarked Anil Okay, Chief Commercial Officer of Alvotech. "We are eager to expand the availability of cost-effective, high-quality biosimilars in the U.S., as they play a crucial role in alleviating inflationary pressures on healthcare providers and patients."
In August 2020, Teva and Alvotech entered into a strategic partnership to exclusively commercialize five of Alvotech's biosimilar candidates. This collaboration was expanded in July 2023 to include two additional biosimilars and new presentations of two previously partnered products. Alvotech manages development and manufacturing, while Teva handles exclusive commercialization in the U.S., utilizing its extensive sales and marketing infrastructure. SIMLANDI is the first product launched under this partnership. Additionally, in April 2024, the FDA approved SELARSDI™ (ustekinumab-aekn) for subcutaneous injection as a biosimilar to Stelara, to treat moderate to severe plaque psoriasis and active psoriatic arthritis in adults and children aged six and older.
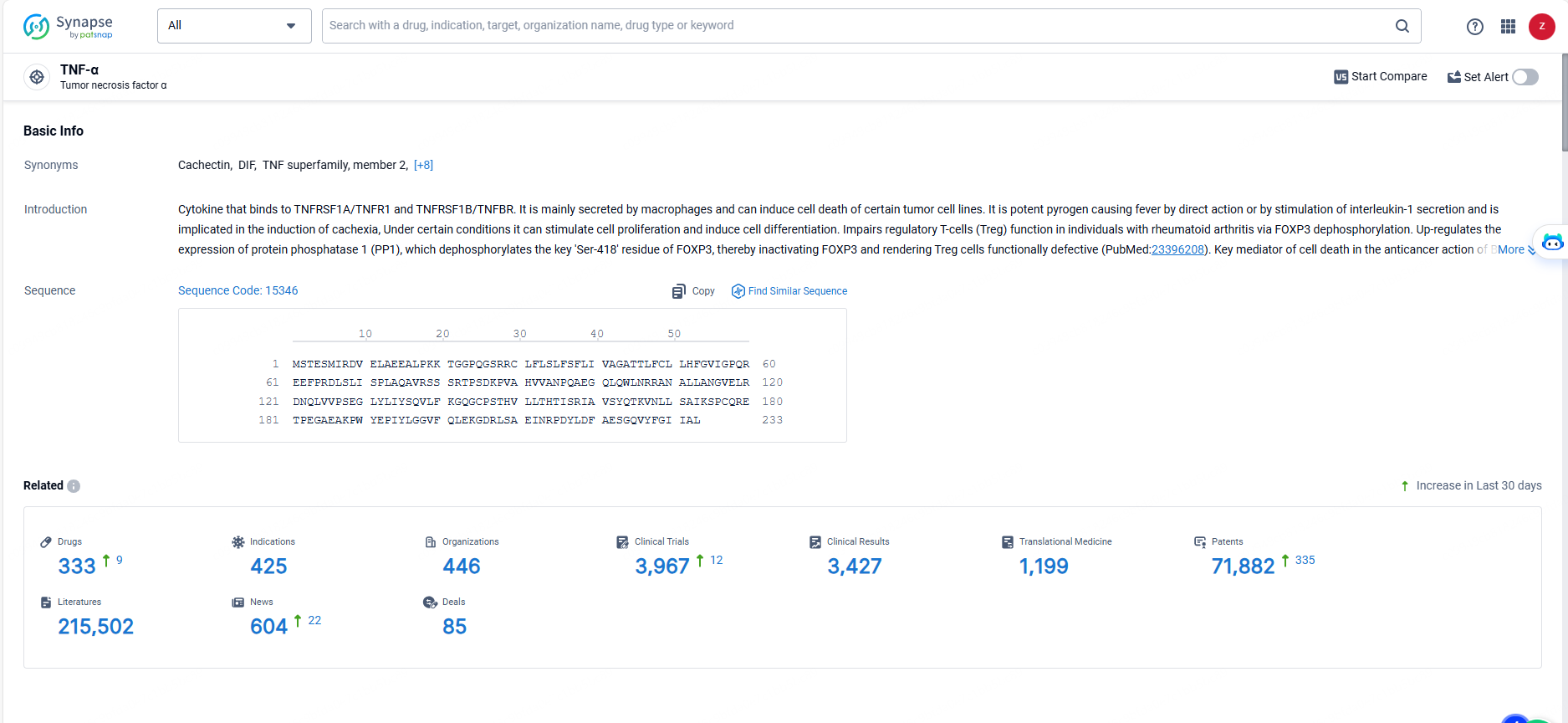
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 333 investigational drugs for the TNF-α protein targets, including 425 indications, 446 R&D institutions involved, with related clinical trials reaching 3967, and as many as 71882 patents.
Adalimumab-ryvk is a monoclonal antibody biosimilar drug that targets TNF-α and is indicated for the treatment of various immune-related and inflammatory conditions. It has received approval in multiple countries, including the European Union, Iceland, Liechtenstein, and Norway, and is used in the treatment of a wide range of therapeutic areas and active indications.