TILT Biotherapeutics Shares Clinical Results of TILT-123 for Ovarian Cancer at ASCO 2024
TILT Biotherapeutics, a company in the clinical-stage biotechnology sector focusing on cancer immunotherapies, has announced its participation at the American Society of Clinical Oncology Annual Meeting 2024 with two abstract presentations. One abstract highlights encouraging safety and efficacy findings of TILT-123 in patients with ovarian cancer, while the other explores the feasibility of TILT-123 as an intravenous treatment option.
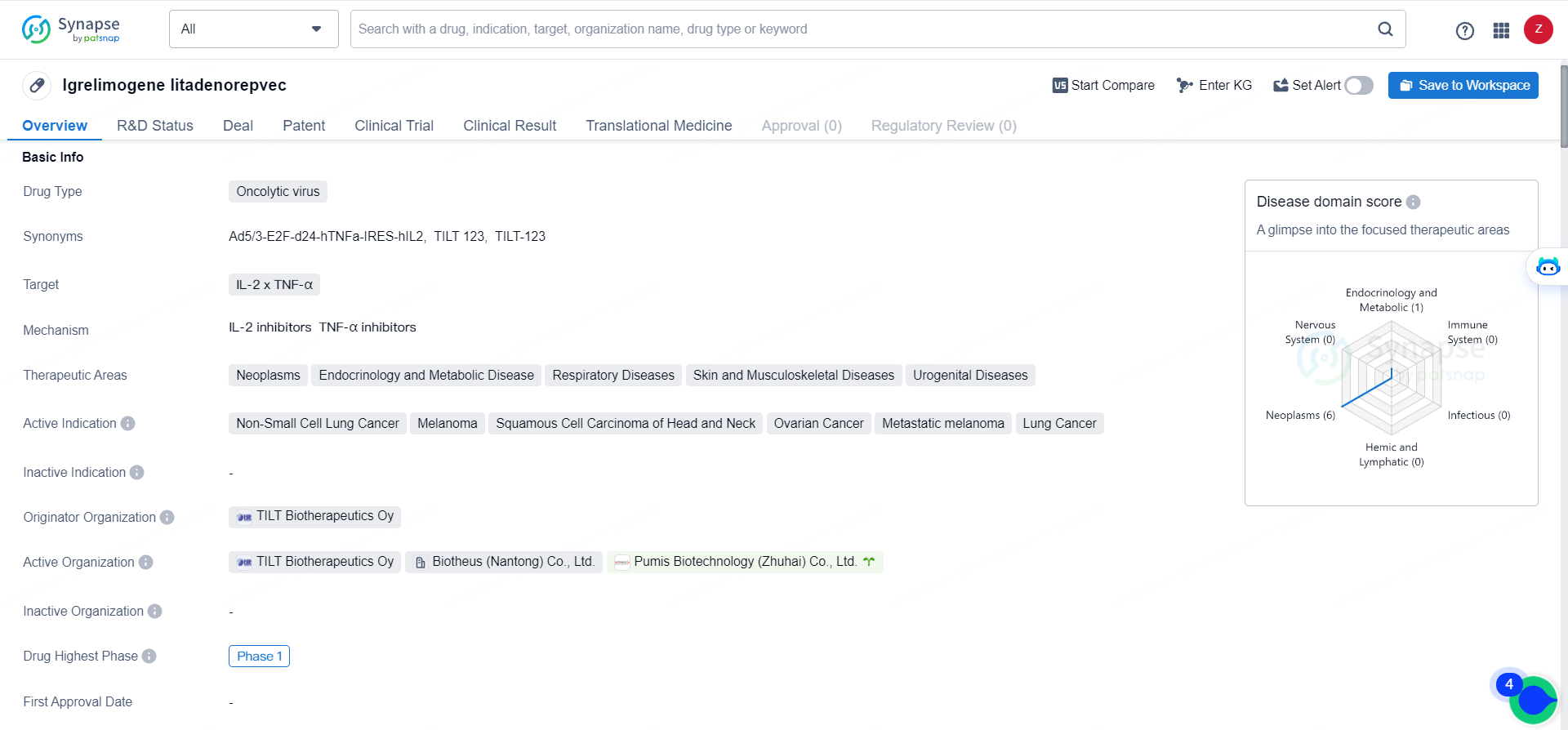
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The outcomes from a Phase I clinical trial involving TILT-123 combined with MSD’s anti-PD-1 therapy KEYTRUDA® (pembrolizumab) for platinum-resistant or -refractory ovarian cancer demonstrate that this combination is safe and shows potential for disease control in a challenging patient group. Among the 14 patients assessable for treatment response, a disease control rate of 64.3% was observed.
Biological sample analysis confirmed the presence of TILT-123 and induction of T cells in both injected and non-injected lesions. Some patients exhibited prolonged survival: median progression-free survival (PFS) and overall survival (OS) for all patients were 105 and 280 days, respectively. For patients exhibiting stable disease by RECIST 1.1 criteria on day 92, median PFS was 174 days and median OS was 293 days.
Across three Phase I trials, intravenous (IV) delivery of TILT-123 resulted in systemic tumor targeting and lymphocyte accumulation in tumors. This signifies that TILT-123 successfully reached and activated an immune response against tumors even without direct injection. The IV administration of TILT-123 led to viral persistence in the peripheral blood for up to 7 days. Tumor transduction was recorded in 75% of the patients by day 8 post IV TILT-123 administration.
Founder and CEO of TILT Biotherapeutics, Akseli Hemminki, a cancer specialist with extensive experience treating cancer patients using oncolytic viruses, remarked, “The data presented at ASCO 2024 strengthens our ongoing clinical trials for patients with resistant or refractory ovarian cancer who have limited treatment alternatives.”
TILT-123, an oncolytic adenovirus equipped with tumor necrosis factor alpha (TNFα) and interleukin-2 (IL-2), aims to boost the effectiveness of T-cell therapies, including immune checkpoint inhibitors and adoptive cell transfer. TILT’s methodology employs oncolytic viruses that selectively reproduce within and destroy cancer cells, while concurrently promoting immune responses against the tumor.
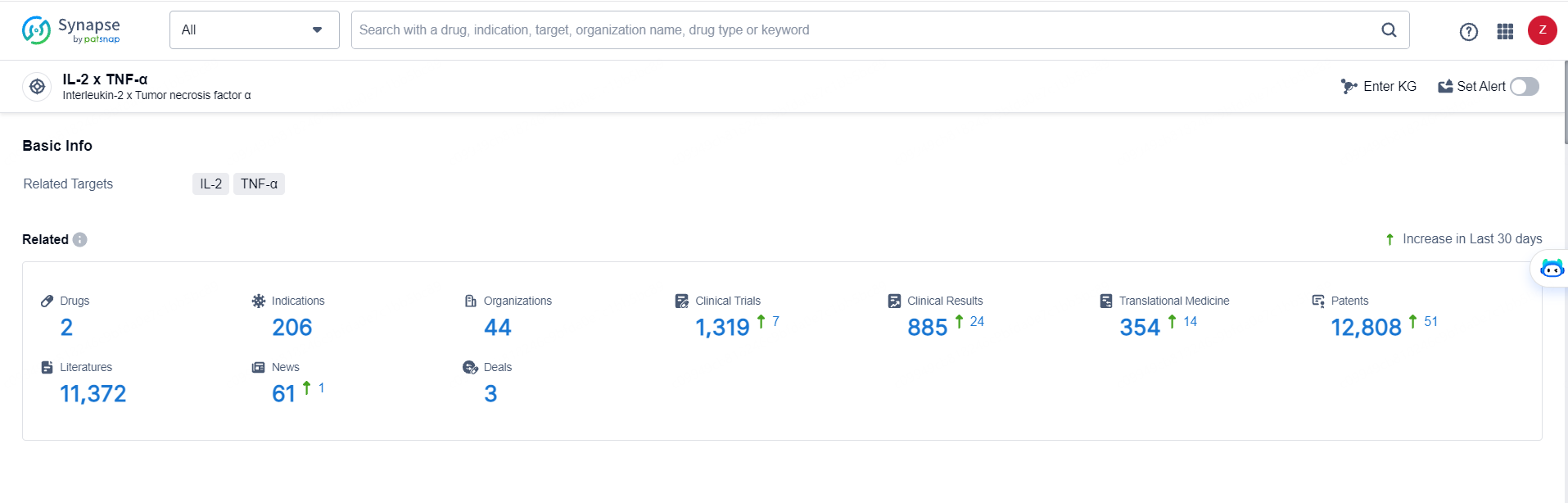
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 29, 2024, there are 2 investigational drugs for the TNFα and IL-2 targets, including 208 indications, 44 R&D institutions involved, with related clinical trials reaching 1319, and as many as 12808 patents.
Igrelimogene litadenorepvec has the potential to make a significant impact in the treatment of cancer, particularly in non-small cell lung cancer, melanoma, squamous cell carcinoma of head and neck, ovarian cancer, metastatic melanoma, and lung cancer. The drug's advancement to Phase 1 in both global and China markets indicates a strong commitment to its development and the potential for it to become a valuable addition to the pharmaceutical industry's arsenal of cancer treatments.