Weekly Insulin Efsitora Alfa Matches Daily Insulin in A1C Reduction: A Fixed Dose Study
Eli Lilly and Company (NYSE: LLY) has announced positive topline results from the QWINT-1 and QWINT-3 phase 3 clinical studies evaluating the weekly insulin efsitora alfa (efsitora) in adults with type 2 diabetes who are either starting basal insulin treatment for the first time (insulin naïve) or switching from daily basal insulin injections. These treat-to-target trials with prolonged duration demonstrated that efsitora accomplished similar A1C reduction compared to the most widely used daily basal insulins globally.
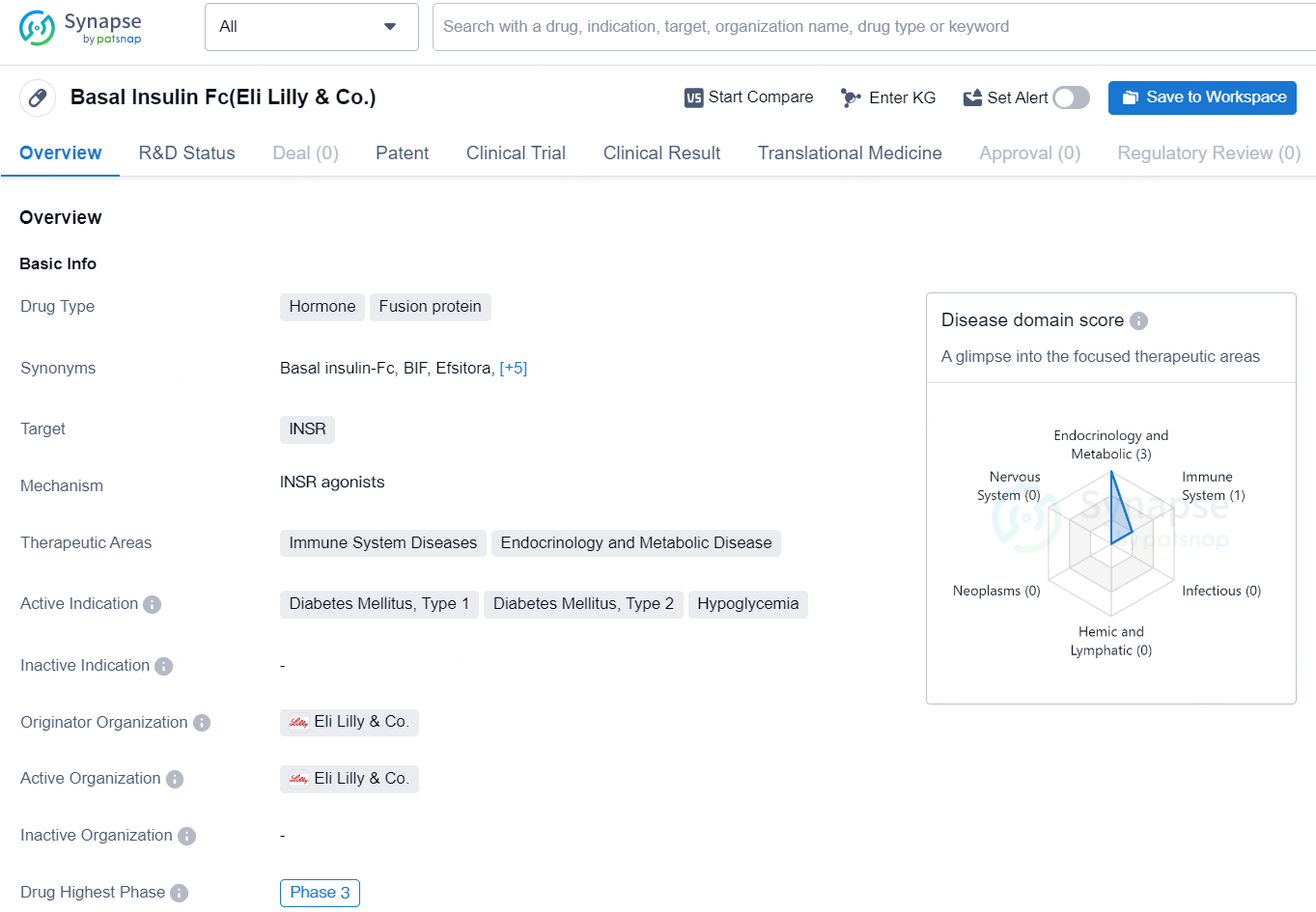
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Efsitora and other insulins designed for weekly use could transform diabetes management," asserted Jeff Emmick, M.D., Ph.D., senior vice president of product development at Lilly. "Many patients find starting insulin therapy overwhelming due to its complexity and inconvenience. A straightforward, fixed-dose weekly regimen with efsitora has the potential to simplify both the initiation and ongoing administration of insulin for diabetics, thus lessening its daily impact."
The QWINT-1 study evaluated the efficacy and safety of weekly-administered efsitora compared to daily insulin glargine over a period of one year. This trial randomized adults with type 2 diabetes, who had not previously used insulin, to receive either a weekly dose of efsitora via a single-use autoinjector or a daily dose of insulin glargine. Efsitora doses were adjusted in four fixed intervals every four weeks based on blood glucose measurements. The study aimed to provide evidence that fixed-dose regimens may simplify the initiation and ongoing management of insulin therapy for diabetic patients.
The trial's primary endpoint was met, showing that efsitora is not inferior to insulin glargine in reducing A1C over 52 weeks. Efsitora achieved an A1C reduction of 1.31%, compared to 1.27% for insulin glargine, with resulting A1C levels of 6.92% and 6.96%, respectively. For the treatment-regimen estimand, efsitora lowered A1C by 1.19% in comparison to 1.16% with insulin glargine, leading to A1C levels of 7.05% versus 7.08%.
QWINT-3 studied the efficacy and safety of once-weekly efsitora compared to once-daily insulin degludec over 78 weeks in adults with type 2 diabetes who were already on basal insulin. Participants were randomly assigned in a ratio of 2:1 to receive either weekly efsitora or daily insulin degludec.
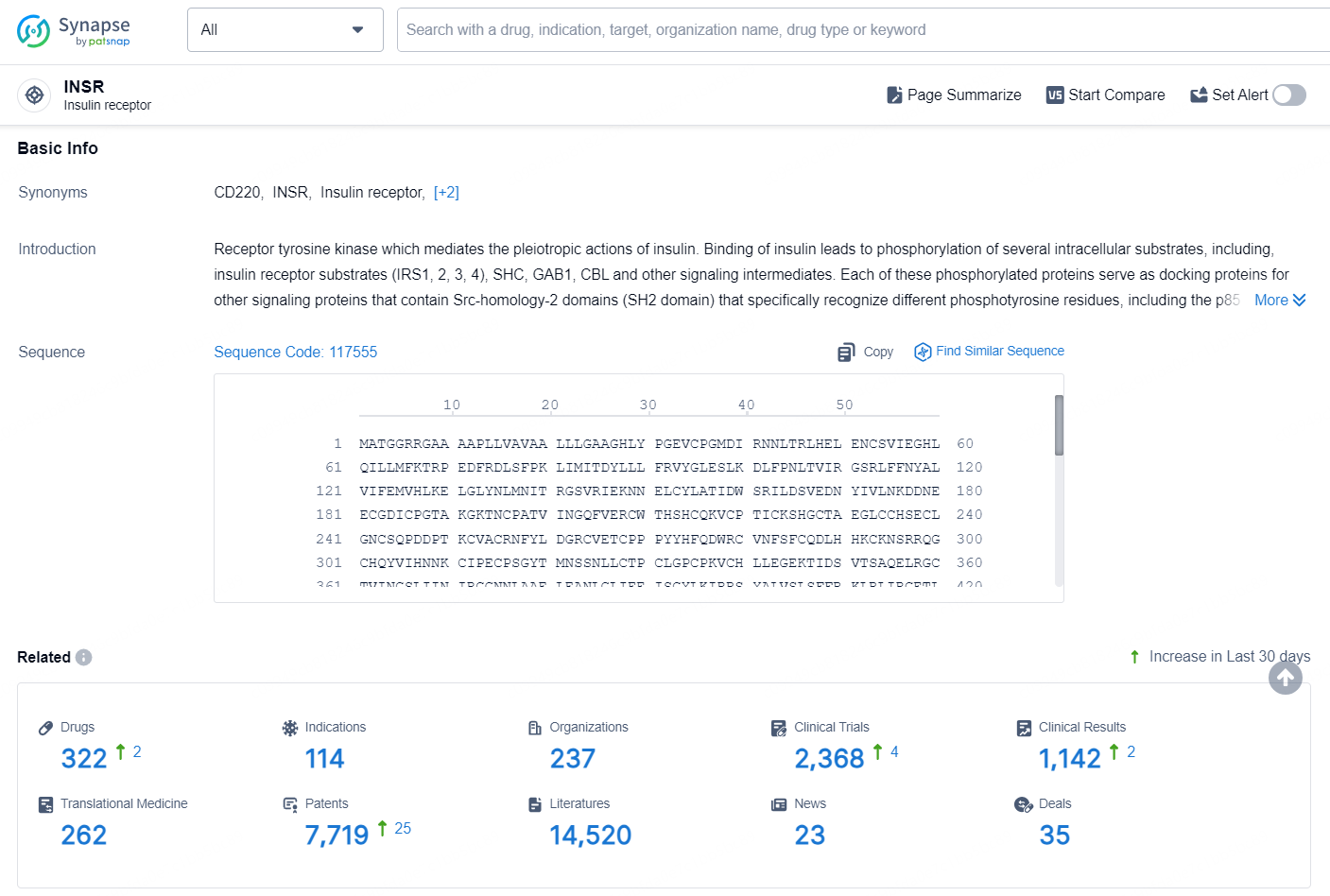
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 9, 2024, there are 322 investigational drugs for the INSR targets, including 114 indications, 237 R&D institutions involved, with related clinical trials reaching 2368, and as many as 7719 patents.
Basal Insulin Fc, developed by Eli Lilly & Co., is a fusion protein hormone that targets the INSR (insulin receptor) and has therapeutic applications in immune system diseases, endocrinology, and metabolic diseases. Its primary indications are for the treatment of Diabetes Mellitus Type 1, Diabetes Mellitus Type 2, and Hypoglycemia.