Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 7-10 November 2022
11 Nov 2022
VaccineDrug ApprovalOrphan Drug
Four new medicines recommended for approvalEMA’s human medicines committee (CHMP) recommended four medicines for approval at its November 2022 meeting.The CHMP recommended authorising the COVID-19 vaccineCOVID-19 vaccine VidPrevtyn Beta (COVID-19 vaccineCOVID-19 vaccine (recombinant, adjuvanted)) as a booster in adults previously vaccinated with an mRNA or adenoviral vector COVID-19 vaccineCOVID-19 vaccine. It is the seventh vaccine recommended in the European Union (EU) for protecting against COVID-19 and, together with the vaccines already authorised, will support vaccination campaigns in EU Member States during the pandemic. See more information in the news announcement in the grid below.The committee adopted a positive opinion for a biosimilar medicine, Kauliv (teriparatide), for the treatment of osteoporosis, a health condition that weakens bones, making them fragile and more likely to break.A generic medicine, Pirfenidone Viatris (pirfenidone), received a positive opinion for the treatment of idiopathic pulmonary fibrosis, a chronic and progressive condition in which the lungs become scarred and breathing becomes increasingly difficult.The CHMP adopted a positive opinion for a generic medicine, Sugammadex Amomed (sugammadex), intended for the reversal of neuromuscular blockade induced by rocuronium in adults and children or vecuronium in adults. Rocuronium and vecuronium are muscle relaxants used during some types of surgeries. Sugammadex is used to speed up the recovery from the effects of the muscle relaxant.Recommendations on extensions of therapeutic indication for 11 medicinesThe committee recommended 11 extensions of indication for medicines that are already authorised in the EU: Ceprotin, Comirnaty, DuoPlavin, Dupixent, Enhertu, Eylea, Imfinzi, Iscover, Lynparza, Plavix and Xofluza.Withdrawals of applicationsTwo applications for marketing authorisation were withdrawn: Orepaxam* for the treatment of pulmonary arterial hypertension, and Febseltiq* for the treatment of cholangiocarcinoma (cancer of the bile ducts).Two applications for extensions of therapeutic indications were withdrawn: Gavreto for the treatment of certain types of thyroid cancer, and Ilaris for the treatment of Schnitzler syndrome, a rare inflammatory disease causing long-term urticaria, recurrent fever, bone and joint pain, and swollen lymph nodes.Question-and-answer documents on the withdrawals are available in the grid below.COVID-19 updateThe committee recommended extending the use of COVID-19 vaccineCOVID-19 vaccine Comirnaty targeting the original strain and Omicron subvariants BA.4 and BA.5 in children between 5 to 11 years of age.An overview of all the COVID-19 vaccinesCOVID-19 vaccines authorised in the EU is available on EMA’s website.Safety updateThe CHMP endorsed the measures recommended by the Pharmacovigilance Risk Assessment Committee (PRAC) to minimise the risk of serious side effects with Janus kinase (JAK) inhibitorsJanus kinase (JAK) inhibitors used to treat several chronic inflammatory disorders. These side effects include cardiovascular conditions, blood clots, cancer and serious infections. This recommendation is the outcome of an article 20 referral procedure, which is triggered for medicines that have been authorised via the centralised procedure in case of quality, safety or efficacy issues. A public health communication on this referral is available in the grid below.Agenda and minutesThe agenda of the November 2022 CHMP meeting is published on EMA's website. Minutes of the October 2022 CHMP meeting will be published in the coming weeks.CHMP statisticsKey figures from the November 2022 CHMP meeting are represented in the graphic below.
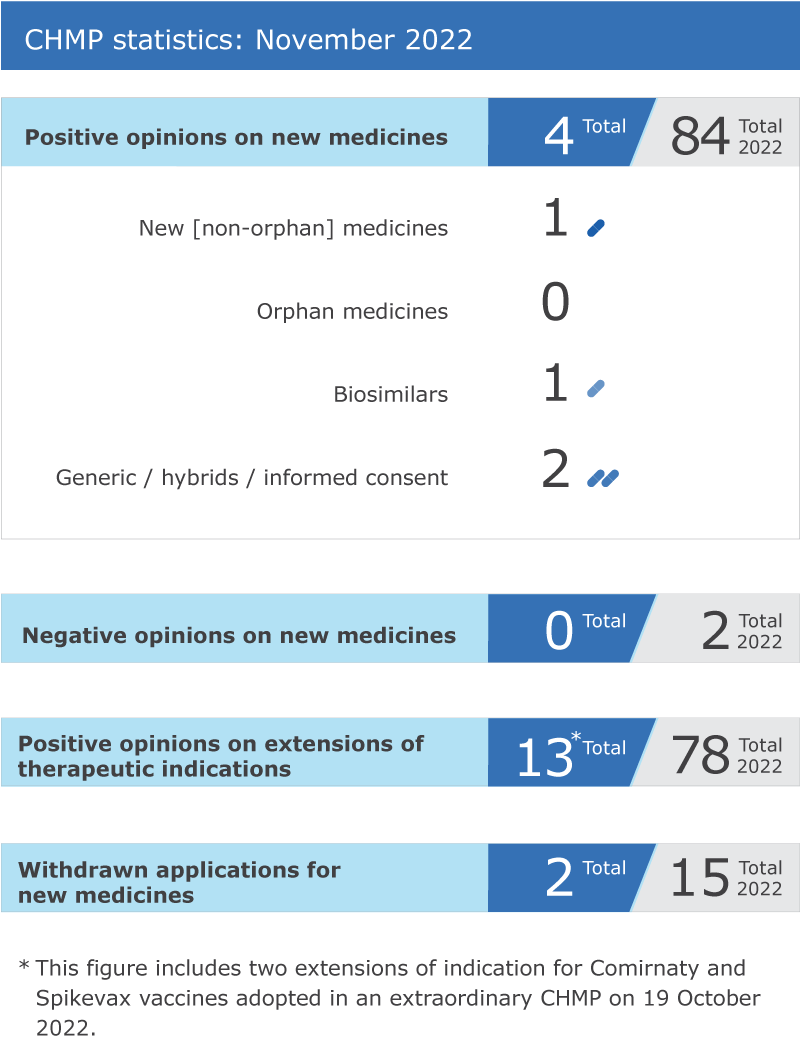
Preview
Source: ema
*This product was designated as an orphan medicine during its development. Orphan designations are reviewed by EMA's Committee for Orphan Medicinal Products (COMP) at the time of approval to determine whether the information available to date allows maintaining the medicine’s orphan status and granting the medicine ten years of market exclusivity.
Positive recommendation on new medicines
Name of medicineVidPrevtyn BetaInternational non-proprietary name (INN)COVID-19 vaccine(INN)COVID-19 vaccine (recombinant, adjuvanted)Marketing-authorisation applicantSanofi PasteurTherapeutic indicationVidPrevtyn Beta is indicated as a booster for active immunisation to prevent COVID-19 in adults who have previously received a mRNA or adenoviral vector COVID-19 vaccineMore informationVidPrevtyn Beta: Pending EC decisionNews announcement:EMA recommends approval of VidPrevtyn Beta as a COVID 19 booster vaccine
Positive recommendation on new biosimilar medicine
Name of medicineKaulivINNteriparatideMarketing-authorisation applicantStrides Pharma CyprusTherapeutic indicationTreatment of osteoporosisMore informationKauliv: Pending EC decision
Positive recommendations on new generic medicines
Name of medicinePirfenidone ViatrisINNpirfenidoneMarketing-authorisation applicantViatris LimitedTherapeutic indicationTreatment of idiopathic pulmonary fibrosisMore informationPirfenidone Viatris: Pending EC decision
Name of medicineSugammadex AmomedINNsugammadexMarketing-authorisation applicantAOP Orphan Pharmaceuticals GmbHTherapeutic indicationReversal of neuromuscular blockade induced by rocuronium or vecuroniumMore informationSugammadex Amomed: Pending EC decision
Positive recommendations on extensions of indications
Name of medicineCeprotinINNhuman protein CMarketing-authorisation holderTakeda Manufacturing Austria AGMore informationCeprotin: Pending EC decision
Name of medicineComirnatyINNtozinameranMarketing-authorisation holderBioNTech Manufacturing GmbHMore informationComirnaty: Pending EC decision
Name of medicineDuoPlavinINNclopidogrel / acetylsalicylic acidMarketing-authorisation holderSanofi-aventis groupeMore informationDuoPlavin: Pending EC decision
Name of medicineDupixentINNdupilumabMarketing-authorisation holderSanofi-aventis groupeMore informationDupixent: Pending EC decision
Name of medicineEnhertuINNtrastuzumab deruxtecanMarketing-authorisation holderDaiichi Sankyo Europe GmbHMore informationEnhertu: Pending EC decision
Name of medicineEyleaINNafliberceptMarketing-authorisation holderBayer AGMore informationEylea: Pending EC decision
Name of medicineImfinziINNdurvalumabMarketing-authorisation holderAstraZeneca ABMore informationImfinzi: Pending EC decision
Name of medicineIscoverINNclopidogrelMarketing-authorisation holderSanofi-aventis groupeMore informationIscover: Pending EC decision
Name of medicineLynparzaINNolaparibMarketing-authorisation holderAstraZeneca ABMore informationLynparza: Pending EC decision
Name of medicinePlavixINNclopidogrelMarketing-authorisation holderSanofi-aventis groupeMore informationPlavix: Pending EC decision
Name of medicineXofluzaINNbaloxavir marboxilMarketing-authorisation holderRoche Registration GmbHMore informationXofluza: Pending EC decision
Withdrawal of initial marketing authorisation application
Name of medicineFebseltiqINNinfigratinibMarketing-authorisation applicantHelsinn Birex Pharmaceuticals LimitedMore informationFebseltiq: Withdrawn application
Name of medicineOrepaxamINNtreprostinil diolamineMarketing-authorisation applicantFerrer Internacional S.A.More informationOrepaxam: Withdrawn application
Withdrawals of post-authorisation marketing authorisation applications
Name of medicineGavretoINNpralsetinibMore informationGavreto: Withdrawn application
Name of medicineIlarisINNcanakinumabMore informationIlaris: Withdrawn application
Conclusion of referral
Name of medicineJanus kinase (JAK) inhibitorsJanus kinase (JAK) inhibitorsMore informationJanus Kinase inhibitors (JAKi)
Other updates
List item
Scientific advice and protocol assistance adopted during the CHMP meeting 7-10 November 2022
(PDF/246.92 KB)
(new)
Adopted
First published:
11/11/2022
EMA/CHMP/SAWP/872304/2022
List item
Start of Union reviews adopted during the CHMP meeting of 7-10 November 2022
(PDF/106.5 KB)
(new)
Adopted
First published:
11/11/2022
EMA/846051/2022
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Indications
Targets
Drugs
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.



