Request Demo
What is Tolvaptan Sodium Phosphate used for?
14 June 2024
Tolvaptan Sodium Phosphate is a pharmaceutical compound that is garnering increasing attention in the medical community for its efficacy in treating particular conditions, notably autosomal dominant polycystic kidney disease (ADPKD). Known by various trade names such as Samsca and Jynarque, Tolvaptan Sodium Phosphate is a selective vasopressin V2-receptor antagonist. This compound is primarily targeted at the kidneys and has been the subject of extensive research conducted by institutions like Otsuka Pharmaceutical Co., Ltd. and several academic medical centers globally. As it stands, Tolvaptan Sodium Phosphate has been approved by the U.S. Food and Drug Administration (FDA) specifically for ADPKD, a genetic disorder characterized by the growth of numerous cysts in the kidneys, which can lead to kidney failure.
Tolvaptan Sodium Phosphate works by inhibiting the action of vasopressin, a hormone that typically promotes water reabsorption in the kidneys. Vasopressin exerts its effect by binding to V2 receptors located in the renal collecting ducts, leading to the insertion of aquaporin-2 water channels into the duct's membrane, thus increasing water reabsorption. By blocking these receptors, Tolvaptan prevents the insertion of aquaporin channels, leading to increased water excretion, or diuresis. This mechanism helps to reduce the rate of cyst growth in patients with ADPKD, thereby slowing the progression of kidney disease. Additionally, by increasing urine output, Tolvaptan can also correct conditions of hyponatremia, where there is an imbalance of sodium levels in the body.
Tolvaptan Sodium Phosphate is typically administered orally in the form of tablets. The drug is taken once or twice daily, depending on the specific needs of the patient and the guidelines set by their healthcare provider. The onset of action for Tolvaptan Sodium Phosphate is relatively rapid, often within a few hours of ingestion. It is important for patients to follow the prescribed dosage regimen carefully and maintain adequate hydration due to the increased urine output caused by the drug.
Like all medications, Tolvaptan Sodium Phosphate comes with a profile of side effects and contraindications. Common side effects include increased thirst, frequent urination, dry mouth, and fatigue. These are generally manageable with proper hydration and lifestyle adjustments. However, more severe side effects can also occur, such as liver injury. Therefore, regular monitoring of liver function through blood tests is recommended for patients on long-term Tolvaptan therapy. Contraindications for the use of Tolvaptan Sodium Phosphate include known hypersensitivity to the drug or its components, as well as conditions such as hyperkalemia or severe dehydration. Pregnant or breastfeeding women should also use this medication only if clearly needed and after consulting with their healthcare provider, as the effects on fetal development and breastfeeding infants remain uncertain.
Several other drugs can interact with Tolvaptan Sodium Phosphate, potentially altering its efficacy or increasing the risk of adverse effects. For instance, inhibitors of the enzyme CYP3A4, such as ketoconazole, fluconazole, and certain antibiotics like clarithromycin and erythromycin, can increase the blood levels of Tolvaptan, thereby raising the risk of toxicity. Conversely, inducers of CYP3A4 like rifampin, carbamazepine, and St. John's Wort can decrease its effectiveness by lowering its blood levels. Patients should inform their healthcare providers about all medications they are currently taking, including over-the-counter drugs, supplements, and herbal products, to avoid potential harmful interactions.
Overall, Tolvaptan Sodium Phosphate represents a significant advancement in the management of conditions such as ADPKD. Its ability to slow the progression of kidney cyst growth offers hope to many patients who face the prospect of deteriorating kidney function. However, like all potent medications, it requires careful management and monitoring to ensure its benefits are maximized while minimizing the risks. As research continues, the medical community will likely gain even deeper insights into its efficacy and safety profile, potentially expanding its use to other conditions characterized by water imbalance or inappropriate vasopressin activity.
Tolvaptan Sodium Phosphate works by inhibiting the action of vasopressin, a hormone that typically promotes water reabsorption in the kidneys. Vasopressin exerts its effect by binding to V2 receptors located in the renal collecting ducts, leading to the insertion of aquaporin-2 water channels into the duct's membrane, thus increasing water reabsorption. By blocking these receptors, Tolvaptan prevents the insertion of aquaporin channels, leading to increased water excretion, or diuresis. This mechanism helps to reduce the rate of cyst growth in patients with ADPKD, thereby slowing the progression of kidney disease. Additionally, by increasing urine output, Tolvaptan can also correct conditions of hyponatremia, where there is an imbalance of sodium levels in the body.
Tolvaptan Sodium Phosphate is typically administered orally in the form of tablets. The drug is taken once or twice daily, depending on the specific needs of the patient and the guidelines set by their healthcare provider. The onset of action for Tolvaptan Sodium Phosphate is relatively rapid, often within a few hours of ingestion. It is important for patients to follow the prescribed dosage regimen carefully and maintain adequate hydration due to the increased urine output caused by the drug.
Like all medications, Tolvaptan Sodium Phosphate comes with a profile of side effects and contraindications. Common side effects include increased thirst, frequent urination, dry mouth, and fatigue. These are generally manageable with proper hydration and lifestyle adjustments. However, more severe side effects can also occur, such as liver injury. Therefore, regular monitoring of liver function through blood tests is recommended for patients on long-term Tolvaptan therapy. Contraindications for the use of Tolvaptan Sodium Phosphate include known hypersensitivity to the drug or its components, as well as conditions such as hyperkalemia or severe dehydration. Pregnant or breastfeeding women should also use this medication only if clearly needed and after consulting with their healthcare provider, as the effects on fetal development and breastfeeding infants remain uncertain.
Several other drugs can interact with Tolvaptan Sodium Phosphate, potentially altering its efficacy or increasing the risk of adverse effects. For instance, inhibitors of the enzyme CYP3A4, such as ketoconazole, fluconazole, and certain antibiotics like clarithromycin and erythromycin, can increase the blood levels of Tolvaptan, thereby raising the risk of toxicity. Conversely, inducers of CYP3A4 like rifampin, carbamazepine, and St. John's Wort can decrease its effectiveness by lowering its blood levels. Patients should inform their healthcare providers about all medications they are currently taking, including over-the-counter drugs, supplements, and herbal products, to avoid potential harmful interactions.
Overall, Tolvaptan Sodium Phosphate represents a significant advancement in the management of conditions such as ADPKD. Its ability to slow the progression of kidney cyst growth offers hope to many patients who face the prospect of deteriorating kidney function. However, like all potent medications, it requires careful management and monitoring to ensure its benefits are maximized while minimizing the risks. As research continues, the medical community will likely gain even deeper insights into its efficacy and safety profile, potentially expanding its use to other conditions characterized by water imbalance or inappropriate vasopressin activity.
How to obtain the latest development progress of all drugs?
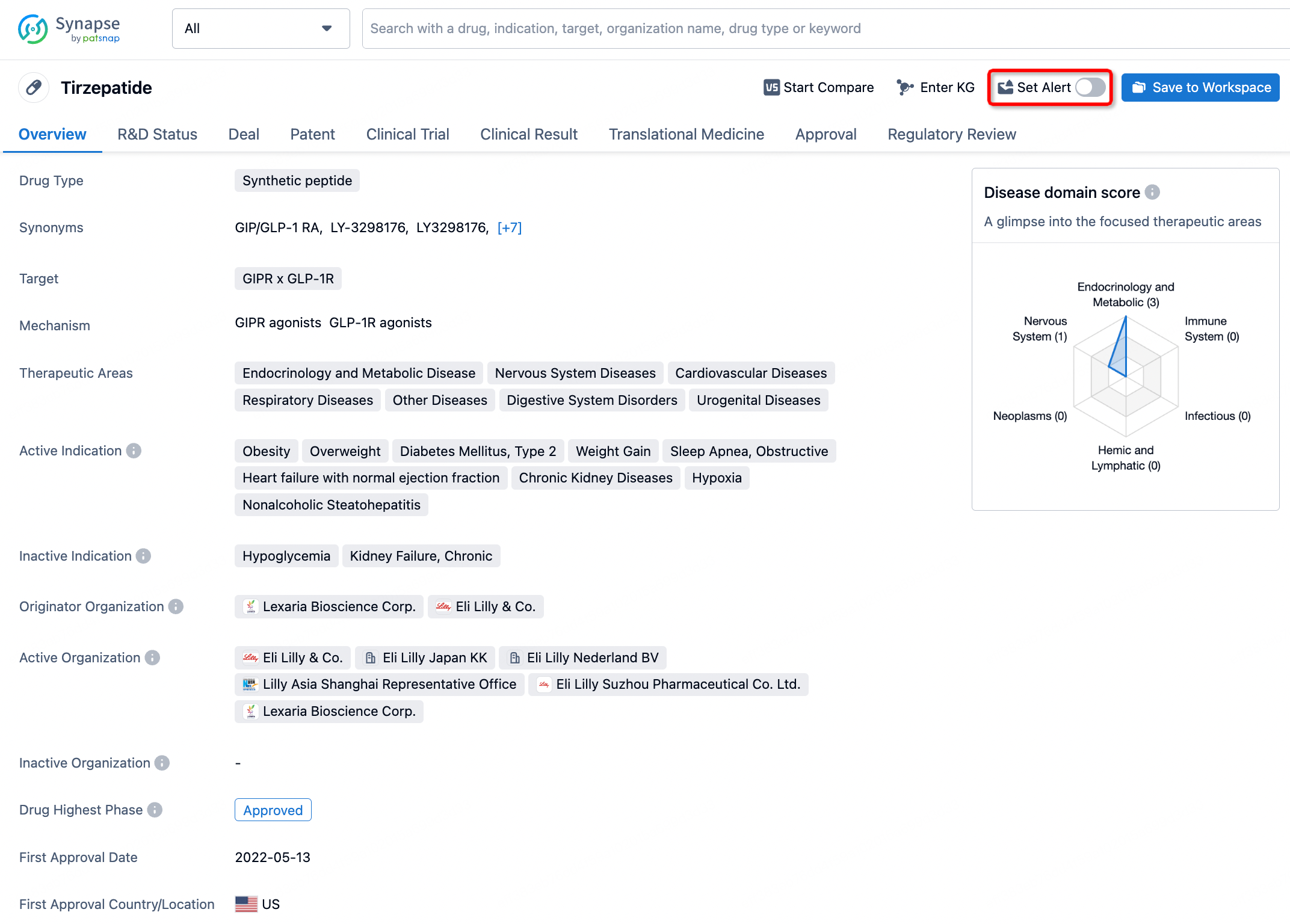
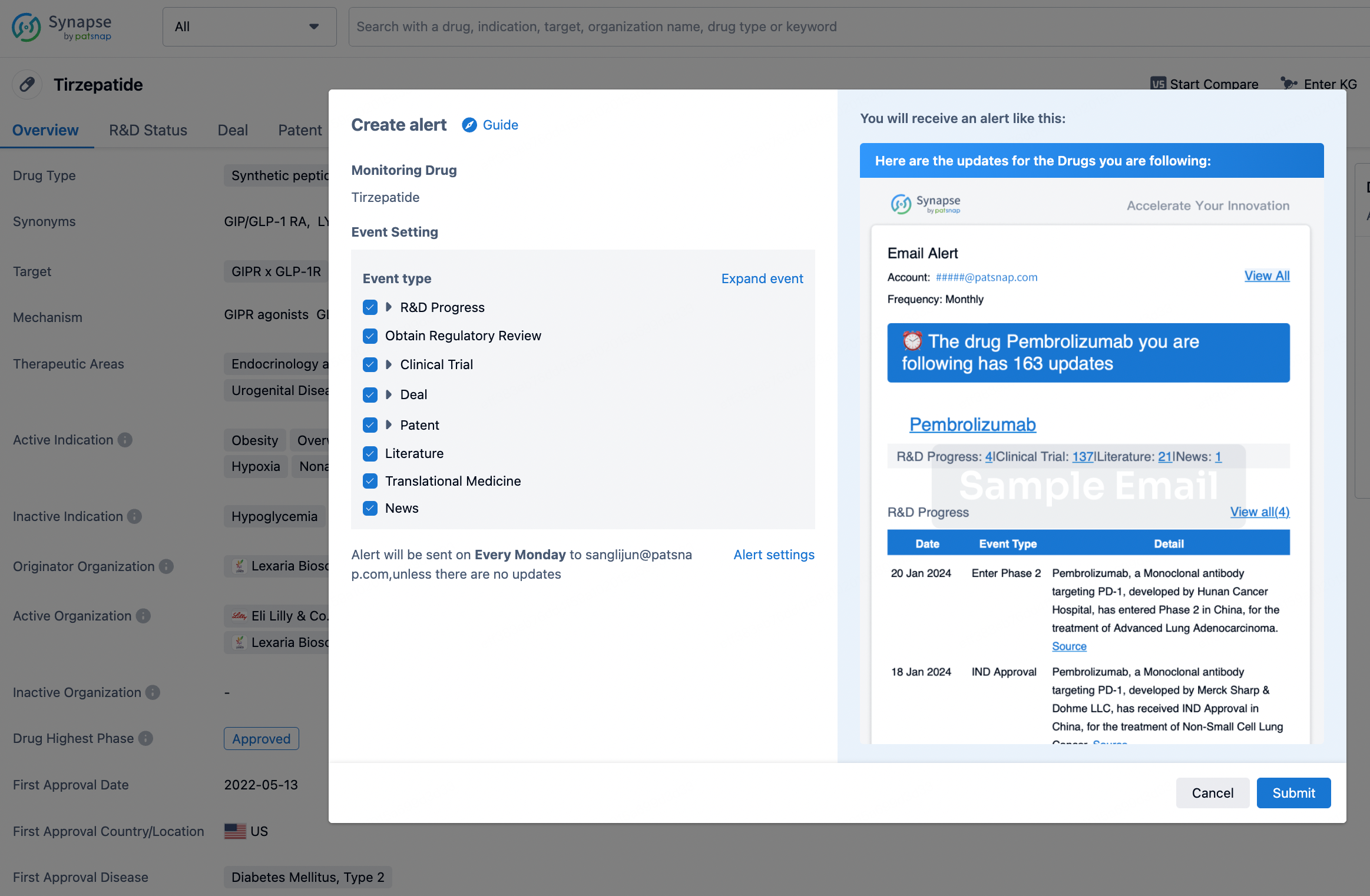
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.