Request Demo
Last update 07 Feb 2026
PEG-Irinotecan
Last update 07 Feb 2026
Overview
Basic Info
Drug Type Polymer |
Synonyms Etirinotecan Pegol, Pegirinotecan, PEGylated irinotecan + [4] |
Target |
Action inhibitors |
Mechanism TOP1 inhibitors(DNA topoisomerase I inhibitors) |
Therapeutic Areas |
Inactive Indication |
Originator Organization |
Active Organization |
Inactive Organization |
License Organization |
Drug Highest PhasePhase 3 |
First Approval Date- |
RegulationOrphan Drug (United States) |
Login to view timeline
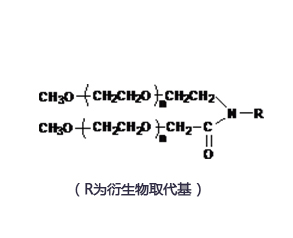
Structure/Sequence
Molecular FormulaC161H192N20O40 |
InChIKeySELCJVNOEBVTAC-UHFFFAOYSA-N |
CAS Registry848779-32-8 |
Related
284
Clinical Trials associated with PEG-IrinotecanNCT07391566
Phase 1b/2 Clinical Study to Evaluate the Safety, Tolerability, Efficacy, and Pharmacokinetics of LPM6690176 Capsules in Combination With Chemotherapy and Bevacizumab in Metastatic Colorectal Cancer Patients With RAS Mutation
This study is consist of phase 1b (dose escalation + safety run-in) and phase 2 (randomized, controlled). Phase 1b is planned to evaluate the safety and tolerability of LPM6690176 capsule in combination with chemotherapy and Bevacizumab in patients with RAS mutant metastatic colorectal cancer (mCRC), to observe the dose-limiting toxicity (DLT), and to determine the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D); Phase 2 is planned to preliminarily evaluate the efficacy of LPM6690176 capsule in combination with chemotherapy + Bev vs. chemotherapy + Bev in patients with previously untreated, RAS mutant mCRC.
Start Date31 Mar 2026 |
Sponsor / Collaborator |
NCT07389265
CAPRI-3 GOIM Study: Phase 3 Clinical Study to Evaluate the Use of Continuing Cetuximab Treatment Beyond First Line Progression in Molecular Selected Metastatic Colorectal Cancer Patients.
The goal of this Phase 3 clinical trial is to evaluate whether continuing cetuximab treatment beyond first-line progression can improve outcomes in patients with metastatic colorectal cancer whose tumors are RAS and BRAF wild-type. The study will compare the effectiveness of chemotherapy given together with cetuximab versus chemotherapy given together with bevacizumab. Researchers aim to determine whether cetuximab continuation improves tumor response, progression-free survival, overall survival, and safety in this patient population.
Eligible participants are adults with metastatic colorectal cancer who have previously responded to first-line treatment with chemotherapy combined with an anti-EGFR antibody. Before starting therapy, patients will undergo molecular testing using liquid biopsy to confirm tumor characteristics. They will then receive chemotherapy with either cetuximab or bevacizumab every two weeks, and their disease will be monitored regularly with CT or MRI scans, laboratory tests, and clinical evaluations. During the study, patients will also provide biological samples for translational research.
This trial will enroll about 360 patients across sites in Italy and Spain and is designed to provide new evidence on whether cetuximab continuation beyond first-line treatment can offer a meaningful clinical benefit compared with standard therapy.
Eligible participants are adults with metastatic colorectal cancer who have previously responded to first-line treatment with chemotherapy combined with an anti-EGFR antibody. Before starting therapy, patients will undergo molecular testing using liquid biopsy to confirm tumor characteristics. They will then receive chemotherapy with either cetuximab or bevacizumab every two weeks, and their disease will be monitored regularly with CT or MRI scans, laboratory tests, and clinical evaluations. During the study, patients will also provide biological samples for translational research.
This trial will enroll about 360 patients across sites in Italy and Spain and is designed to provide new evidence on whether cetuximab continuation beyond first-line treatment can offer a meaningful clinical benefit compared with standard therapy.
Start Date01 Oct 2025 |
Sponsor / Collaborator |
NCT07312422
An Open-label, Single-center, Single-arm Study to Evaluate the Efficacy and Safety of Low-dose Radiation Therapy Combined With Pultelimab and Standard Treatment in Patients With Advanced Pancreatic Cancer
To investigate the activating effect of local lesion low-dose radiotherapy (2Gy) on the tumor immune microenvironment, and the efficacy, safety, and feasibility of its combination with pembrolizumab and standard therapy in patients with advanced pancreatic cancer. Concurrently, to preliminarily establish an efficacy prediction model for the early identification of patient populations who would benefit from the treatment, thereby providing a theoretical foundation for the implementation of precision medicine.
Start Date30 Sep 2025 |
Sponsor / Collaborator |
100 Clinical Results associated with PEG-Irinotecan
Login to view more data
100 Translational Medicine associated with PEG-Irinotecan
Login to view more data
100 Patents (Medical) associated with PEG-Irinotecan
Login to view more data
45
Literatures (Medical) associated with PEG-Irinotecan01 Dec 2025·LANCET
Preoperative mFOLFIRINOX versus PAXG for stage I–III resectable and borderline resectable pancreatic ductal adenocarcinoma (PACT-21 CASSANDRA): results of the first randomisation analysis of a randomised, open-label, 2 × 2 factorial phase 3 trial
Article
Author: Di Marco, Mariacristina ; Palumbo, Diego ; Sperti, Elisa ; Balzano, Gianpaolo ; Orsi, Giulia ; Liscia, Nicole ; Macchini, Marina ; Merelli, Barbara ; Scartozzi, Mario ; Lonardi, Sara ; Bencardino, Katia ; Carconi, Catia ; Bozzarelli, Silvia ; Rapposelli, Ilario Giovanni ; Malleo, Giuseppe ; Ercolani, Giorgio ; Tamburini, Emiliano ; Falconi, Massimo ; Belfiori, Giulio ; Torri, Valter ; Tamburrino, Domenico ; Milella, Michele ; Mazzola, Michele ; Procaccio, Letizia ; Reni, Michele
BACKGROUND:
Perioperative chemotherapy is a standard option for treatment of patients with resectable and borderline resectable pancreatic ductal adenocarcinoma (PDAC). This study aimed to assess the superiority of PAXG (cisplatin, nab-paclitaxel, capecitabine, and gemcitabine) over mFOLFIRINOX (modified fluorouracil, leucovorin, irinotecan, and oxaliplatin) in this population.
METHODS:
CASSANDRA is a randomised, open-label, 2 × 2 factorial phase 3 trial, involving 17 Italian academic hospitals. Eligible patients were aged 18-75 years with pathologically confirmed resectable or borderline resectable PDAC. Randomisation was performed by a central web-based system using R-code lists with a computerised algorithm. The design adopted a 1:1 randomisation, with a block stratification by centre and carbohydrate antigen 19-9. Participants were first randomly assigned PAXG (total daily capecitabine dose of 1250 mg/m2 in a 625 mg/m2 twice a day dosage and intravenous cisplatin 30 mg/m2, nab-paclitaxel 150 mg/m2, and gemcitabine 800 mg/m2 every 14 days) or mFOLFIRINOX (intravenous fluorouracil 2400 mg/m2, leucovorin 400 mg/m2, irinotecan 150 mg/m2, and oxaliplatin 85 mg/m2 every 14 days) for 4 months, followed by a second randomisation to 2 months of additional chemotherapy either before or after surgery. The primary endpoint was event-free survival (EFS) in the intention-to-treat population and the safety population included all patients who received at least one cycle of the assigned therapy. The results of the first randomisation are reported here. The trial, registered on ClinicalTrials.gov (NCT04793932) and EudraCT (2020-003080-26 and 2024-519031-42-00), completed accrual and reached the necessary events for first randomisation primary analysis but follow-up of overall survival is ongoing.
FINDINGS:
Between Nov 3, 2020, and April 24, 2024, 132 eligible patients were assigned to PAXG and 128 to mFOLFIRINOX. In the PAXG group, the median age was 65 years (IQR 60-70), 68 (52%) of 132 patients were female, and 64 (48%) were male. In the mFOLFIRNOX group, the median age was 63 years (IQR 57-69), 62 (48%) of 128 patients were female, and 66 (52%) were male. All 260 patients received at least one assigned chemotherapy administration. PAXG prolonged the median EFS compared with mFOLFIRINOX (16·0 months [95% CI 12·4-19·8] vs 10·2 months [8·6-13·5]; hazard ratio 0·63 [0·47-0·84]; p=0·0018). At least one grade 3 or worse adverse event was observed in 87 (66%) of 132 patients in the PAXG group and in 78 (61%) of 128 patients in the mFOLFIRINOX group, including one fatal event.
INTERPRETATION:
PAXG significantly improved EFS compared with mFOLFIRINOX in resectable or borderline resectable PDAC. Preopertive PAXG could be considered a standard option for resectable or borderline resectable PDAC. Accordingly, preoperative PAXG should be considered as the standard comparator group for future trials in this setting.
FUNDING:
MyEverest and Codice Viola.
01 Jun 2024·Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.]
A real-world analysis of second-line treatment option, gemcitabine plus anlotinib and anti-PD1, in advanced pancreatic cancer
Article
Author: Si, Haiyan ; Deng, Guochao ; Ma, Yue ; Dai, Guanghai ; Jia, Ru ; Fan, Mengjiao ; Wang, Zhikuan
BACKGROUND:
In the second-line treatment of advanced pancreatic cancer (APC), there is only one approved regimen based on the phase III NAPOLI-1 trial. However, for patients progressing after Nab-paclitaxel and Gemcitabine (Nab-P/Gem) or Nab-P combinations, second-line treatment were very limited.
METHODS:
This is a retrospective single-center analysis of patients. Our aim was to determine the effectiveness and tolerability of a novel regimen, gemcitabine plus Anlotinib and anti-PD1, in APC patients and to compare it with oxaliplatin, irinotecan, leucovorin, and fluorouracil (FOLFIRINOX) in the second-line setting who have failed on the first-line Nab-P combinations.
RESULTS:
In total, twenty-three patients received Gemcitabine plus Anlotinib and anti-PD1 in the second-line, 28 patients were treated with FOLFORINOX. There was no significant difference in overall survival (OS) or progression free survival (PFS) for either of the two sequences (p > 0.05). Patients who received Gemcitabine plus Anlotinib and anti-PD1 had a median PFS of 4.0 months (95% CI: 1.1-6.9) versus 3.5 months (95% CI 1.8-5.2) in FOLFORINOX group (p = 0.953). The median OS of Gemcitabine plus Anlotinib and anti-PD1 was 9.0 months (95% CI: 4.0-13.7) and 8.0 months (95% CI: 5.5-10.5) in FOLFORINOX group (p = 0.373). Grade ≥3 treatment-emergent adverse events (AEs) occurred for 13% of patients with Gemcitabine plus Anlotinib and anti-PD1 and 40% for FOLFORINOX.
CONCLUSION:
Our data confirms the effectiveness of Gemcitabine plus Anlotinib and anti-PD1 as a well-tolerated regimen in the second-line treatment of APC and extends available data on its use as a second-line treatment option when compared with FOLFIRINOX.
01 Nov 2022·JAMA oncology
Etirinotecan Pegol Treatment for Patients With Metastatic Breast Cancer and Brain Metastases—Reply
Letter
Author: Tolaney, Sara M. ; Tagliaferri, Mary ; Tripathy, Debu
16
News (Medical) associated with PEG-Irinotecan11 Sep 2024
Median overall survival of 15.6 months in Phase 2 Bria-IMT™ study patients treated in combination with immune checkpoint inhibitorOS of 15.6 months compares favorably with 6.7-9.3 months reported for similar patients in the literatureOngoing Phase 3 study investigating Bria-IMT™ in similar metastatic breast cancer populationNo drug related discontinuations to date PHILADELPHIA and VANCOUVER, British Columbia, Sept. 11, 2024 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW) (TSX: BCT) (“BriaCell” or the “Company”), a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care, is pleased to announce positive overall survival data of its Phase 2 clinical study of Bria-IMT™ in combination with an immune check point inhibitor (CPI) in late stage metastatic breast cancer. Median overall survival of 15.6 months is reported in BriaCell’s most recent patients (treated since 2022) vs. 6.7-9.3 months for similar patients reported in the literature (see table below). These patients are being treated with the same Bria-IMT™ formulation currently being used in BriaCell’s ongoing Phase 3 pivotal study in metastatic breast cancer (listed on ClinicalTrials.gov as NCT06072612) and represent patients enrolled post-COVID when full study activities resumed. This represents a substantial improvement over BriaCell’s 13.4 months median overall survival previously reported in December 2023. “Overall survival in patients with heavily pre-treated metastatic breast cancer is very poor,” stated Sara A. Hurvitz, MD, Professor of Medicine, Fred Hutch Cancer Center and University of Washington and BriaCell medical advisory board member. “The BriaCell early data is quite encouraging from both efficacy and tolerability standpoints.” “We wanted to look at the Phase 2 data of those patients who most closely resemble the patients being treated in our ongoing phase 3 study and compare them to similar patients in the literature,” stated Dr. William V. Williams, BriaCell’s President and CEO. “The nearly two-fold overall survival benefit we are seeing with the Bria-IMT™ regimen, together with the similar previously reported approximate doubling of progression free survival, compared with literature controls, strongly support our belief that Bria-IMT™ could have a meaningful impact in the lives of heavily pre-treated metastatic breast cancer patients. We look forward to further clinical development of Bria-IMT™ with the goal of establishing it as a new standard of care for patients with metastatic breast cancer.” “The Bria-IMT™ regimen is the only investigational drug we have seen to show these impressive survival numbers in heavily pre-treated metastatic breast cancer patients who have failed numerous prior treatments including immune check point inhibitors and antibody drug conjugates,” stated Giuseppe Del Priore, MD, MPH, BriaCell’s Chief Medical Officer. “These survival and clinical benefit data support BriaCell’s hypothesis of additive and/or synergistic effects of immune check point inhibitors with Bria-IMT™ and drive the ongoing pivotal study of our combination regimen in the treatment of metastatic breast cancer.” The Phase 2 study enrolled 54 heavily pre-treated metastatic breast cancer patients (average number of prior treatments = 6) who were treated with the Bria-IMT™ regimen and an immune checkpoint inhibitor. Of these 54 patients, 37 were treated with the Phase 3 formulation and 25 of these were treated post-COVID when full study activities resumed. This data represents an additional six months of follow-up of the survival data presented at the San Antonio Breast Cancer Symposium in December 2023. Table 1. Comparative Median Overall Survival (OS) and Progression-Free Survival (PFS) in Similar Patients (Interim Analysis Using Kaplan-Meier Estimate) StudyPrior Lines of Therapy (median, range)Number of PatientsOS (months)PFS (months) BriaCell’s Phase 2 study patients who received pivotal Phase 3 study formulation (since 2022)5.5 (2-13)2515.64.1 BriaCell’s Phase 2 study patients who received pivotal Phase 3 study formulation (total)6 (2-13)3713.43.9 Bardia, A. et. al. 1 (TNBC)4 (2-14)2626.91.7 Tripathy D. et. al. 2 (Brain metastases)≥4 in 91%1787.5-7.81.9-2.8 O’Shaughnessy J. et. al. 3non-TNBC at initial diagnosis5 (2-14)766.72.3 O’Shaughnessy J. et. al. 3TNBC at initial diagnosis4 (2-10)1576.91.6 Cortes et. al. 44 (0-13)5949.1-9.31.9-2.5
References Bardia A, et al. Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression. J Clin Oncol. 2024 May 20;42(15):1738-1744. doi: 10.1200/JCO.23.01409. Epub 2024 Feb 29. PMID: 38422473.Tripathy D, et al. Treatment with etirinotecan pegol for patients with metastatic breast cancer and brain metastases: final results from the phase 3 ATTAIN randomized clinical trial. JAMA Oncol. 2022;8(7):1047-1052. doi:10.1001/jamaoncol.2022.0514.O’Shaughnessy J et al. Analysis of patients without and with an initial triple-negative breast cancer diagnosis in the phase 3 randomized ASCENT study of sacituzumab govitecan in metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2022 Sep;195(2):127-139. doi: 10.1007/s10549-022-06602-7. Epub 2022 May 11. PMID: 35545724; PMCID: PMC9374646.Cortes J, Perez-Garcia J, Levy C, Gómez Pardo P, Bourgeois H, Spazzapan S, Martínez-Jañez N, Chao TC, Espié M, Nabholtz JM, Gonzàlez Farré X, Beliakouski V, Román García J, Holgado E, Campone M. Open-label randomised phase III trial of vinflunine versus an alkylating agent in patients with heavily pretreated metastatic breast cancer. Ann Oncol. 2018 Apr 1;29(4):881-887. doi: 10.1093/annonc/mdy051. PMID: 29481630. About BriaCell Therapeutics Corp. BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/. Safe Harbor This press release contains “forward-looking statements” that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” “will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements, including statements about: the impact of Bria-IMT™ on patients with metastatic breast cancer; BriaCell’s further clinical development of Bria-IMT™; and the efficacy of immune check point inhibitors, are based on BriaCell’s current expectations and are subject to inherent uncertainties, risks, and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading “Risks and Uncertainties” in the Company’s most recent Management’s Discussion and Analysis, under the heading “Risk Factors” in the Company’s most recent Annual Information Form, and under “Risks and Uncertainties” in the Company’s other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company’s profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law. Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release. Contact Information Company Contact:William V. Williams, MDPresident & CEO1-888-485-6340info@briacell.com Media Relations:Jules AbrahamCORE IRjulesa@coreir.com Investor Relations Contact:CORE IRinvestors@briacell.com
Phase 3ImmunotherapyPhase 2Clinical Result
18 Jul 2024
Progression free survival (PFS) extended to 9.1 months in ADC resistant patient - quadruple the PFS of patients in similar studies 1, 2, 3Significant reduction of “Eye-Bulging” metastatic breast cancer tumor was previously reportedHeavily pre-treated patient had failed 8 prior regimens including antibody-drug conjugate (ADC) therapy and continues to receive BriaCell treatment
PHILADELPHIA and VANCOUVER, British Columbia, July 18, 2024 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW) (TSX: BCT) (“BriaCell” or the “Company”), a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care, is pleased to report significantly higher PFS for its top responder patient in the Phase 2 study of BriaCell’s Bria-IMT™ regimen in combination with an immune checkpoint inhibitor in metastatic breast cancer. The patient remains alive and she continues to receive BriaCell’s treatment regimen.
“We are extremely pleased with the unprecedented survival benefit in this very-difficult-to-treat patient,” stated Dr. William V. Williams, BriaCell’s President and CEO. “This data represents a step forward in our efforts to build on our knowledge and successes to transform cancer care for patients. We expect to replicate this positive data in our ongoing Phase 3 study and bring relief to cancer patients whose medical needs remain unmet.”
“Despite recent advances in cancer therapy, metastatic breast cancer remains an unmet medical need, as current treatments are limited by poor survival and harsh side effects,” commented Dr. Giuseppe Del Priore, BriaCell’s Chief Medical Officer. “The Bria-IMT™ regimen produced a much longer than expected survival benefit in addition to its favorable safety and tolerability in this patient suggesting its potential as a therapeutic option for these cancer patients.”
The patient had a large right orbital lesion (behind the right eye) and a right temporal lobe lesion (in the right side of the brain). The temporal lobe lesion is no longer detectable, while the orbital lesion has continued to shrink markedly (see figure showing resolution of proptosis post treatment (small arrows) with reduction in tumor indicated by the large arrows). In addition, her tumor markers (blood tests that correlate with the amount of tumor in the body) have markedly decreased from her pre-treatment levels.
Figure 1: Responder Images - Bria-IMT™ Regimen
Table 1: Bria-IMT™ and historical clinical data in MBC patients who failed multiple prior treatments StudyPFS (months)Top Responder Patient9.1+Bardia, A. et. al. 11.7Tripathy D. et. al. 21.9O’Shaughnessy J. et. al. non-TNBC 32.3O’Shaughnessy J. et. al. TNBC 31.6
1,2,3 Data is shown for the intent to treat population for the control group treated with treatment of physician’s choice, which is the comparator in the BriaCell’s ongoing pivotal Phase 3 study.2 This paper describes patients with brain metastases, which were also present in the patient described.+ Indicates the patient is ongoing in the study.
References
Bardia A, et al. Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression. J Clin Oncol. 2024 May 20;42(15):1738-1744. doi: 10.1200/JCO.23.01409. Epub 2024 Feb 29. PMID: 38422473.Tripathy D, et al. Treatment with etirinotecan pegol for patients with metastatic breast cancer and brain metastases: final results from the phase 3 ATTAIN randomized clinical trial. JAMA Oncol. 2022;8(7):1047-1052. doi:10.1001/jamaoncol.2022.0514.O’Shaughnessy J et al. Analysis of patients without and with an initial triple-negative breast cancer diagnosis in the phase 3 randomized ASCENT study of sacituzumab govitecan in metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2022 Sep;195(2):127-139. doi: 10.1007/s10549-022-06602-7. Epub 2022 May 11. PMID: 35545724; PMCID: PMC9374646.
About BriaCell Therapeutics Corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/.
Safe Harbor
This press release contains “forward-looking statements” that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” “will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements, including those about BriaCell replicating positive data in its ongoing Phase 3 study; BriaCell’s Bria-IMT™ regimen bringing relief to cancer patients whose medical needs remain unmet; and the Bria-IMT™ regimen becoming a therapeutic option for metastatic breast cancer patients, are based on BriaCell’s current expectations and are subject to inherent uncertainties, risks, and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading “Risks and Uncertainties” in the Company’s most recent Management’s Discussion and Analysis, under the heading “Risk Factors” in the Company’s most recent Annual Information Form, and under “Risks and Uncertainties” in the Company’s other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company’s profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:William V. Williams, MDPresident & CEO1-888-485-6340info@briacell.com
Media Relations:Jules AbrahamCORE IRjulesa@coreir.com
Investor Relations Contact:CORE IRinvestors@briacell.com
A photo accompanying this announcement is available at:https://www.globenewswire.com/NewsRoom/AttachmentNg/cef51e5b-ff25-4c3e-929d-22ad6df22927
ImmunotherapyPhase 2Phase 3Clinical ResultADC
03 Jun 2024
BriaCell doubles Progression-Free-Survival (PFS) and Clinical Benefit Rate vs historical results in the literatureBria-IMT™ PFS compares favorably to PFS of most recent treatment in 48% of Antibody-Drug Conjugate (ADC) resistant patientsTherapy well-tolerated with no Bria-IMT™ related discontinuationsClinical data highlight significant potential of Bria-IMT™ in advanced metastatic breast cancerSuperiority of selected Phase 3 regimen and formulation confirmedOral presentation by Mayo Clinic Professor and Principal Investigator, Saranya Chumsri, MD, on Monday June 3; 11:30 AM-1:00 PM CDT PHILADELPHIA and VANCOUVER, British Columbia, June 03, 2024 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW) (TSX: BCT) (“BriaCell” or the “Company”), a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care, announces positive clinical efficacy data updates of its ongoing randomized Phase 2 study evaluating lead clinical candidate Bria-IMT™ in patients with advanced metastatic breast cancer. Two poster sessions, one abstract, and one oral presentation session (by Principal Investigator and Professor of Oncology, Mayo Clinic, Saranya Chumsri, MD), will be presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting taking place today June 3, 2024 at McCormick Place, Chicago, IL. “We are very impressed with both clinical efficacy and safety data in these heavily pretreated patients. Given the limited effective treatment options in this group of patients, and the fact that most treatments are associated with significant toxicities, physicians and patients often opt to decline further ineffective and toxic drugs in lieu of palliative care,” Saranya Chumsri, MD, Principal Investigator and Professor of Oncology, Mayo Clinic. “Antibody-drug conjugates (ADCs) and immune checkpoint Inhibitors (CPIs) have emerged as the latest therapies to treat these patients. However, a large percentage of late-stage patients do not respond, and all patients inevitably develop resistance to them, making a safe and effective treatment an urgent medical need. BriaCell’s novel immunotherapy offers a well-tolerated treatment option for these patients beyond the currently approved drugs.” “While immunotherapy has emerged as an active treatment option for multiple cancer types, its use in breast cancer is rather restricted to a minority of patients. Discovering new strategies, in order to enhance the responsiveness of various subtypes of breast cancer to immunotherapy, presents as a therapeutic opportunity. Through its unique mechanism of action, Bria-IMT™ regimen selectively activates adaptive cancer-fighting CD4+ and CD8+ T cells and innate responses (dendritic and NK cells) to activate patients’ immune systems without producing serious side effects,” stated Carmen Calfa, M.D., Clinical Research Lead for the breast site disease group at the University of Miami Miller School of Medicine, Co-Director of the Cancer Survivorship Program at Sylvester Comprehensive Cancer Center, and Principal Clinical Investigator of the Phase 2 Bria-IMT™ study. “Our ASCO presentations highlight how Bria-IMT™’s activities – through diverse mechanisms including adaptive and innate responses - synergize with multiple mechanisms of action of checkpoint inhibitors,” stated Dr. Williams, BriaCell’s President and CEO. “We believe Bria-IMT™ has the potential to become a breakthrough novel treatment option for patients in advanced metastatic breast cancer.” The details of the presentations are summarized below. Oral Presentation Summary Abstract Number for Publication: 1022Title: Outcomes of advanced/metastatic breast cancer (aMBC) treated with Bria-IMT™, an allogeneic whole cell immunotherapy.Session Type and Title: Rapid Oral Abstract – Breast Cancer—MetastaticSession Date and Time: 6/3/2024; 11:30 AM-1:00 PM CDT This presentation details the results of BriaCell’s randomized Phase 2 study of Bria-IMT™ in combination with retifanlimab, an immune checkpoint inhibitor (CPI). The goal of randomization was to compare whether administration of the CPI early, in the first cycle of therapy, or later, late in the second cycle of therapy, offered any advantage. Two different formulations of Bria-IMT™ were also evaluated; one treated with interferon gamma and one untreated. The patients entering the study were very heavily pretreated and had failed multiple prior therapies as shown in the Table 1 below. Table 1. Prior Therapies in the Bria-IMT™ Phase 2 Study Previous TherapiesNumber of Patients (%)Antibody-Drug Conjugates (ADC)23 (44%)Immune Checkpoint Inhibitor (CPI)11 (20%)Cyclin-Dependent Kinase (CDK) 4/6 Inhibitors34 (63%) A total of 54 patients were included in the Phase 1/2 study. Nearly half of these had been treated previously with an antibody drug conjugate and had progressed in their disease following this treatment. Another 20% had failed a prior immune checkpoint inhibitor. Nearly 2/3 of the patients had failed therapy with a CDK 4/6 inhibitor. On average they had failed six prior therapy attempts. In the Phase 2 portion of the study, there were 32 patients with 16 treated with CPI early and 16 treated with CPI late. There was no statistically significant difference in progression-free survival (PFS) two groups. However, a slight advantage in the CPI early group has led this to be the selected regimen for the Phase 3 study. In the entire Phase 1/2 experience, with 54 patients, the formulation not incubated with interferon gamma showed a statistically significant improvement in PFS. Therefore, this formulation was selected for the Phase 3 study. The data are shown in Figure 1. Figure 1. Effect of treatment sequence and formulation on PFS Clinical benefit was seen in 55% of evaluable patients across all subtypes of breast cancer as shown in Figure 2 below. Figure 2: Objective Response Rate (ORR) and Clinical Benefit Rate (CBR) in the Bria-IMT™ Phase 1/2 Study The progression free survival rate and the clinical benefit rate as well as the objective response rate were markedly higher than those of similar patients treated with the treatment of their physician’s choice in other studies. Notably, “Treatment of Physician’s Choice” (TPC) will be the comparator in the Phase 3 study of Bria-IMT™. This is noted in Table 2 below. Table 2. Comparative PFS, ORR and CBR in Similar Patients StudyPrior Lines ofTherapy(median, range)PFS(months)ORR(%)CBR(%)BriaCell's Phase 2 study patients who received pivotal Phase 3 study formulation 6 (2-13)3.99.5*55*BriaCell's ADC Resistant Phase 2 patients who received pivotal Phase 3 study formulation 6 (3-13)4.112**53**Bardia, A. et. al. 14 (2-14)1.748Tripathy D. et. al. 2≥4 in 91%1.9310 O'Shaughnessy J. et. al. non-TNBC 35 (2-14)2.347 O'Shaughnessy J. et. al. TNBC 34 (2-10)1.6510 *Data is for evaluable patients, n=42 with 12 not evaluable. ** Data is for evaluable patients, n = 17 with 6 not evaluable.References: Data is shown for the intent to treat population for the control group treated with treatment of physician’s choice, which is the comparator in the BriaCell Phase 3 study1. Bardia A, et al. J Clin Oncol. 2024 May 20;42(15):1738-1744. 2. Tripathy D, et al. JAMA Oncol. 2022 Nov 1;8(11):1700-1701. jamaoncol.2022.4346. PMID: 36136348. This paper describes patients with brain metastases.3. O'Shaughnessy J, et al. Breast Cancer Res Treat. 2022 Sep;195(2):127-139. For additional detailed information of the clinical data on the oral presentation, please visit BriaCell Doubles Progression-Free-Survival (PFS) and Reports Clinical Benefit Data at ASCO 2024. Poster Presentation Summary The first poster described BriaCell’s ongoing pivotal Phase 3 registrational study in advanced metastatic breast cancer. BriaCell is excited to collaborate on this important program with authors and BriaCell medical advisory board members Sara A. Hurvitz, MD, Professor of Medicine, Fred Hutchinson Cancer Center, Adam M. Brufsky, MD, PhD, Professor of Medicine, University of Pittsburgh School of Medicine, and Massimo Cristofanilli, MD, Professor of Medicine, Weill Cornell Medical College, Cornell University. The second poster described clinical data of Bria-IMT™ in metastatic breast cancer patients who failed antibody drug conjugates (ADCs) and is spearheaded by Chaitali Nangia, MD, Partner, Hoag Medical Group, and Carmen Calfa, MD, Professor of Medicine, University of Miami.Abstract Number for Publication: TPS1137Title: Study of the Bria-IMT™ regimen and CPI vs physicians' choice in advanced metastatic breast cancer (BRIA-ABC).Based on Phase 2 clinical data showing numerous survival and clinical benefit outcomes in advanced breast cancer patients treated with the Bria-IMT™ regimen, the pivotal Phase 3 study has been designed as a multicenter randomized, open label comparison of the Bria-IMT™ regimen plus CPI in one arm versus Treatment of Physicians' Choice (TPC) in metastatic breast cancer patients with no approved alternative therapies available. Patients’ eligibility includes treatment with 2 or more prior regimens. There will be another arm of the Bria-IMT™ regimen alone (monotherapy). For additional information on the pivotal Phase 3 study, please visit ClinicalTrials.gov as NCT06072612. Abstract Number for Publication: 1087Title: SV-BR-1-GM after progression on ADC in patients with metastatic breast cancer. Remarkable progression-free survival and clinical benefit of Bria-IMT™ in ADC resistant advanced metastatic breast cancer Phase 2 clinical data of the Bria-IMT™ regimen in 23 advanced metastatic breast cancer patients who failed multiple prior treatments including ADCs and CPIs (median of 6 prior treatments) are presented. Clinical efficacy In evaluable patients, the ORR was 12% and CBR was 53% which is remarkable versus similar data suggesting clinical benefit.Median PFS of 4.1 months with the Phase 3 formulation was ~twice that seen of patients in similar studies - 1.71 and 2.23 months - who received TPC. The PFS results suggest superior clinical efficacy given the larger number of prior treatments (median of 6) in Bria-IMT™ patients vs those of the other studies (median of 4).Subset specific clinical benefits: Study data to date suggests clinical benefit for multiple breast cancer subtypes including HR+/HER2- (the most common breast cancer subtype, testing positive for estrogen and/or progesterone receptors and negative for human epidermal growth factor receptor 2 or HER2) with a CBR following treatment, of 63% (5 of 8 patients); HER2+ subtype (a positive test for HER2) with a 100% CBR (2 of 2 patients) and HR-/HER2 low subtype (a negative test for estrogen and/or progesterone receptor and a negative test for HER2) showing a CBR of 66% (2 of 3 patients). See Table 3. Table 3: Treatment Efficacy by Metastatic Breast Cancer Subtype in ADC-resistant patients HistologyAll Patients (N)Evaluable (N) PatientsBest ORRBest CBRAll ADC Resistant231712% (2 / 17)53% (9 / 17)ER/PR + / HER2 low or -8813% (1 / 8)63% (5 / 8)HER2+3250% (1 / 2)100% (2 / 2)TNBC127029% (2 / 7) Bria-IMT™ showed potential survival advantage over penultimate treatment in 48% of patients, likely by reversing immune exhaustion in patients irrespective of specific prior ADC. Safety profile Absence of both interstitial lung disease (ILD), a common serious adverse event with ADCs, and Bria-IMT™-related treatment discontinuations underscore Bria-IMT™'s excellent tolerability and favorable safety profile. In summary, the data to date shows that Bria-IMT™ offers extended progression-free survival and clinical benefit in heavily pre-treated, ADC resistant breast cancer patients versus those in other similar studies. BriaCell is closely monitoring ADC resistant patients in its ongoing pivotal Phase 3 study of Bria-IMT™ and CPI in advanced metastatic breast cancer. Title: Differential efficacy of SV-BR-1-GM in inducing intracranial metastasis regression. Superior clinical benefit of Bria-IMT™ regimen - alone or combined with an immune check point inhibitor (CPI) in advanced breast cancer patients with CNS metastatic disease Central nervous system (CNS) metastases, including brain metastases and other intracranial metastases, is a dire clinical situation with very poor survival. Very few therapies have shown any effect on CNS or intracranial metastases in breast cancer and it is a serious unmet medical need. Clinical efficacy: 83% (5/6) intracranial objective response rate (iORR) was reported in evaluable patients with central nervous system (CNS) metastases treated with the Bria-IMT™ regimen, either alone or in combination with an immune checkpoint inhibitor (i.e. PD-1 inhibitor pembrolizumab or retifanlimab). These patients failed multiple prior treatments including 2 antibody-drug conjugates in one case. This is illustrated in Figure 3. Figure 3. Intracranial Tumor Responses in Patients with Intracranial Metastases Treated with Bria-IMT™ Tumor reductions (≥30% reduction in the sum of diameters) were observed in heavily pretreated patients highlighting potential clinical benefit of Bria-IMT™ in managing CNS metastasesThis is a pre-planned subgroup analysis in the pivotal Phase 3 study of Bria-IMT™ providing another opportunity for approval Safety profile: No treatment related discontinuation was reported. In summary, Bria-IMT™’s tumor reductions observed in patients with intracranial disease underlines its potential clinical effectiveness in managing CNS metastatic disease in advanced breast cancer. BriaCell will continue to monitor the data in this subgroup of patients in its ongoing pivotal Phase 3 study in advanced metastatic breast cancer. Treatment of patients with CNS metastatic disease represents a potential additional indication for market approval of Bria-IMT™. Copies of the poster presentations and abstracts are posted on https://briacell.com/scientific-publications/. References Bardia A, et al. Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression. J Clin Oncol. 2024 May 20;42(15):1738-1744. doi: 10.1200/JCO.23.01409. Epub 2024 Feb 29. PMID: 38422473.Tripathy D, et al. Treatment with etirinotecan pegol for patients with metastatic breast cancer and brain metastases: final results from the Phase 3 ATTAIN randomized clinical trial. JAMA Oncol. 2022;8(7):1047-1052. doi:10.1001/jamaoncol.2022.0514.O'Shaughnessy J et al. Analysis of patients without and with an initial triple-negative breast cancer diagnosis in the Phase 3 randomized ASCENT study of sacituzumab govitecan in metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2022 Sep;195(2):127-139. doi: 10.1007/s10549-022-06602-7. Epub 2022 May 11. PMID: 35545724; PMCID: PMC9374646. About BriaCell Therapeutics Corp. BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/. Safe Harbor This press release contains “forward-looking statements” that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” “will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements, including those about the presentation at the 2024 ASCO of two poster sessions, one abstract, and the delivery of an oral presentation by Dr. Saranya Chumsri, and the contents of all such materials and presentations; BriaCell's novel immunotherapy offering a well-tolerated treatment option for patients beyond the currently approved drugs; Bria-IMT™ having the potential to become a breakthrough novel treatment option for patients in advanced metastatic breast cancer; “Treatment of Physician’s Choice” (TPC) being the comparator in the Phase 3 study of Bria-IMT™; monotherapy becoming another arm of the Bria-IMT™ regimen; the potential clinical benefit of Bria-IMT™ in managing CNS metastases disease in advanced breast cancer; BriaCell continuing to monitor the data in the intracranial disease subgroup of patients in its ongoing pivotal Phase 3 study in advanced metastatic breast cancer; and the treatment of patients with CNS metastatic disease representing a potential additional indication for market approval of Bria-IMT™, are based on BriaCell’s current expectations and are subject to inherent uncertainties, risks, and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading “Risks and Uncertainties” in the Company’s most recent Management’s Discussion and Analysis, under the heading “Risk Factors” in the Company’s most recent Annual Information Form, and under “Risks and Uncertainties” in the Company's other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company's profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law. Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release. Contact Information Company Contact:William V. Williams, MDPresident & CEO1-888-485-6340info@briacell.com Media Relations:Jules AbrahamCORE IRjulesa@coreir.com Investor Relations Contact:CORE IRinvestors@briacell.com Photos accompanying this announcement are available at https://www.globenewswire.com/NewsRoom/AttachmentNg/6fff10ca-4660-4c6f-bb46-719f0f21887dhttps://www.globenewswire.com/NewsRoom/AttachmentNg/2b7cbddb-da76-486d-b6c3-22894a42933ahttps://www.globenewswire.com/NewsRoom/AttachmentNg/66f03119-a4b3-4d2d-a998-ab355aefdeac
ImmunotherapyClinical ResultASCOPhase 3Phase 2
100 Deals associated with PEG-Irinotecan
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| D10367 | PEG-Irinotecan |
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Extensive stage Small Cell Lung Cancer | Phase 3 | China | 29 Sep 2024 | |
| Recurrent Lung Small Cell Carcinoma | Phase 3 | - | 16 Sep 2024 | |
| Brain metastases | Phase 3 | United States | 01 Nov 2016 | |
| Brain metastases | Phase 3 | Australia | 01 Nov 2016 | |
| Brain metastases | Phase 3 | Belgium | 01 Nov 2016 | |
| Brain metastases | Phase 3 | Canada | 01 Nov 2016 | |
| Brain metastases | Phase 3 | France | 01 Nov 2016 | |
| Brain metastases | Phase 3 | Israel | 01 Nov 2016 | |
| Brain metastases | Phase 3 | Italy | 01 Nov 2016 | |
| Brain metastases | Phase 3 | Portugal | 01 Nov 2016 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 1 | 9 | mFOLFIRINOX (Dose Level 1) | xueywxoedn = nfrrfccvns kwtbrvyzjh (ekfsoftlwl, kqauzghnxv - wfruxxgruz) View more | - | 24 Oct 2024 | ||
mFOLFIRINOX (Dose Level 2) | xueywxoedn = demqnjbcvx kwtbrvyzjh (ekfsoftlwl, szsdhbludo - vutabufkyw) View more | ||||||
Phase 2 | 19 | Stereotactic Body Radiation+Leucovorin Calcium+irinotecan+oxaliplatin+Irinotecan hydrochloride+FLUOROURACIL (Cohort 1: SBRT and FOLFIRINOX) | knfuanxsyr = lkaguvxfgl ihgewuosyz (zfsglwncmm, avlkjrpkxg - gjcebpfzfc) View more | - | 14 Aug 2024 | ||
Stereotactic Body Radiation (Cohort 2: SBRT and Modified FOLFIRINOX) | knfuanxsyr = bfjhysfrug ihgewuosyz (zfsglwncmm, hwtmgisavh - tvhlthtqsq) View more | ||||||
Phase 2 | 34 | ntwahidkgs(suowrzwgyw) = kskghddwzf kgrmlmbtau (rvhmtetqtn, ofxermdyud - ytjtqefnoy) View more | - | 14 Aug 2024 | |||
ntwahidkgs(suowrzwgyw) = sqqyvocdcv kgrmlmbtau (rvhmtetqtn, yeiwptvrqx - lstlvixatc) View more | |||||||
Phase 2 | 276 | yytlsyavsm = ehkujlistl dforracdqo (umavbppiea, lucgnljatk - ctqlimaaez) View more | - | 10 Jul 2024 | |||
(FOLFIRINOX + LV5FU2 in Maintenance) | yytlsyavsm = dbabvvvlce dforracdqo (umavbppiea, trchredlxl - spidsawcux) View more | ||||||
Phase 2 | 5 | Stereotactic Body Radiation Therapy+Leucovorin Calcium+irinotecan+oxaliplatin+Irinotecan hydrochloride+FLUOROURACIL | jdnmcdmsos = mldhbsttoq hzzrjqzuqc (swajatipir, cuswvfdozt - dlvoiitqtl) View more | - | 05 Mar 2024 | ||
Phase 2 | Extensive stage Small Cell Lung Cancer Second line | 29 | enkuwfkmyz(ghwpfmhncf) = mgleljlhag aqsiqihlyp (nkowpwveih ) View more | Positive | 27 Sep 2023 | ||
Phase 2 | 9 | fcrsipyvls = zqeffobezz wsvivgyddu (ugeixkodhg, kbhwodojqy - dwkkkuywvs) View more | - | 27 Oct 2022 | |||
Phase 1 | 14 | (FOLFIRINOX or Gemcitabine/Abraxane Followed by SBRT Dose Level 1) | dcnxswdnbq(bbaomrksai) = zobyalsdec eujcmmrtyr (rmsyiedibb, icksaeiqne - bpzqarmvsv) View more | - | 13 Oct 2022 | ||
(FOLFIRINOX or Gemcitabine/Abraxane Followed by SBRT Dose Level 2) | dcnxswdnbq(bbaomrksai) = mzyprxdstp eujcmmrtyr (rmsyiedibb, yljgfihtcw - vqpixtsoar) View more | ||||||
Phase 2 | 126 | mFOLFIRINOX (Arm 1 (mFOLFIRINOX + Surgery + FOLFOX)) | ngiibdgmjx = pdzrxgdvem uvqnnviqyh (fyocylfdws, jwphdebjgh - dsoqtqwoee) View more | - | 24 May 2022 | ||
FOLFOXx (Arm 2 (mFOLFIRINOX + Radiation + Surgery + FOLFOXx)) | ngiibdgmjx = sftqvgtaoi uvqnnviqyh (fyocylfdws, uhexemcpiq - ppzzqjiiuh) View more | ||||||
Phase 3 | 178 | jxpoypryyv(vslhgkctzs) = gcprgoftjm zxhkxzpxzc (tgkvewmiee ) View more | Negative | 12 May 2022 | |||
Chemotherapy (eribulin, ixabepilone, vinorelbine, gemcitabine, paclitaxel, docetaxel, or nab-paclitaxel) | jxpoypryyv(vslhgkctzs) = hdptojtpwy zxhkxzpxzc (tgkvewmiee ) View more |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free