Request Demo
Pharmaceutical Insights
Navigate pharmaceutical trends with our insights on targets, institutional pipelines, clinical advances, and new drugs.

Recent blog posts
Latest Hotspot
3 min read
Initial Dosing in Phase I Glioblastoma Trial by ITM, Helmholtz Munich, and University Hospital Münster
25 April 2024
ITM, Helmholtz Munich, and University Hospital Münster Report Initial Dosing of First Participant in Phase I Research Trial for Glioblastoma.
Hot Spotlight
10 min read
Progress in the Research of Therapeutic Peptides Targeting GPCRs
25 April 2024
GPCRs, as key signal transduction molecules on the cell surface, regulate various physiological processes and constitute one-third of the targets for clinical drug applications.
Latest Hotspot
3 min read
EU Sanctions Voydeya as Supplementary Treatment for PNH with Persistent Haemolytic Anaemia
25 April 2024
Voydeya has been sanctioned in the EU as a supplementary treatment alongside ravulizumab or eculizumab for adults with PNH who still suffer from persistent haemolytic anaemia.
"What" Series
3 min read
What is LD50?
25 April 2024
LD50 (median lethal dose) is a commonly used indicator in toxicology to describe the toxicity of toxic substances or radiation.
Latest Hotspot
3 min read
The Phase 3 LUNA 3 trial of Rilzabrutinib successfully achieved its main goal in treating immune thrombocytopenia
25 April 2024
The recent phase 3 LUNA 3 trial demonstrated that 400 mg of rilzabrutinib taken orally twice daily effectively achieved the primary goal of sustaining platelet response in adults with persistent or chronic immune thrombocytopenia.
Latest Hotspot
3 min read
FDA Approves ImmunityBio’s ANKTIVA® for BCG-Resistant Bladder Cancer
25 April 2024
ImmunityBio Receives FDA Nod for ANKTIVA®, a Novel IL-15 Receptor Stimulator, for Treating BCG-Resistant Non-Muscle Invasive Bladder Cancer.
Latest Hotspot
3 min read
Neurogene to Present Early Results on NGN-401 Gene Therapy for Rett Syndrome at ASGCT Conference
25 April 2024
Neurogene Set to Reveal NGN-401 Gene Therapy Safety Results from Early Stage Trial for Rett Syndrome during ASGCT Conference.
Latest Hotspot
3 min read
Adicet Bio Showcases Preclinical Readiness of ADI-270 for IND at 27th ASGCT Meeting
25 April 2024
Adicet Bio presents preclinical findings indicating IND preparedness for ADI-270 during a talk at the 27th Annual ASGCT Meeting.
Pharma Frontiers
11 min read
Pharma Frontiers: Daily Digest of Global Pharmaceutical News - April 25
25 April 2024
April 25th latest updates in the global new drug development field, including progress in new drug research and development, transaction information, and partnership developments.
Latest Hotspot
3 min read
ASLAN Pharmaceuticals Announces Positive Early Results from Phase 2 Eblasakimab Trial in Atopic Dermatitis Patients Previously on Dupilumab
24 April 2024
ASLAN Pharmaceuticals Reports Encouraging Preliminary Outcomes from a Phase 2 Trial of Eblasakimab in Patients with Atopic Dermatitis Previously Treated with Dupilumab.
Graphic Pharma
19 min read
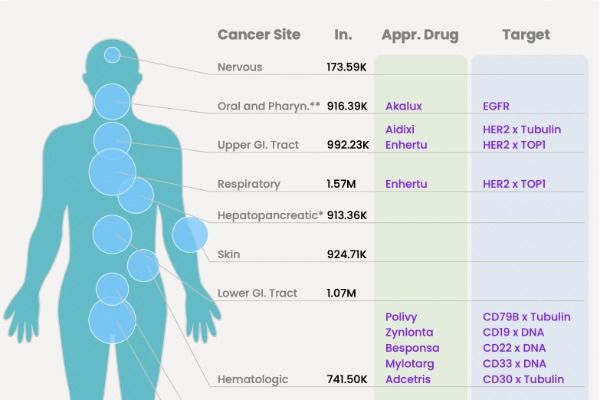
Compilation of 13 FDA-Approved ADC (Antibody-Drug Conjugate) Medications
24 April 2024
At present, 13 types of ADCs have been approved by the United States Food and Drug Administration (FDA), with over 200 additional ADCs at various stages of clinical trials.
Latest Hotspot
3 min read
AGA China Phase II Study GT20029 Successfully Meets Its Primary Goal
24 April 2024
Kintor Pharmaceutical Limited announced that its innovative proteolysis targeting chimera compound, GT20029, targeting the androgen receptor (AR), has achieved the primary endpoint in a phase II trial in China.